Scientific Achievement
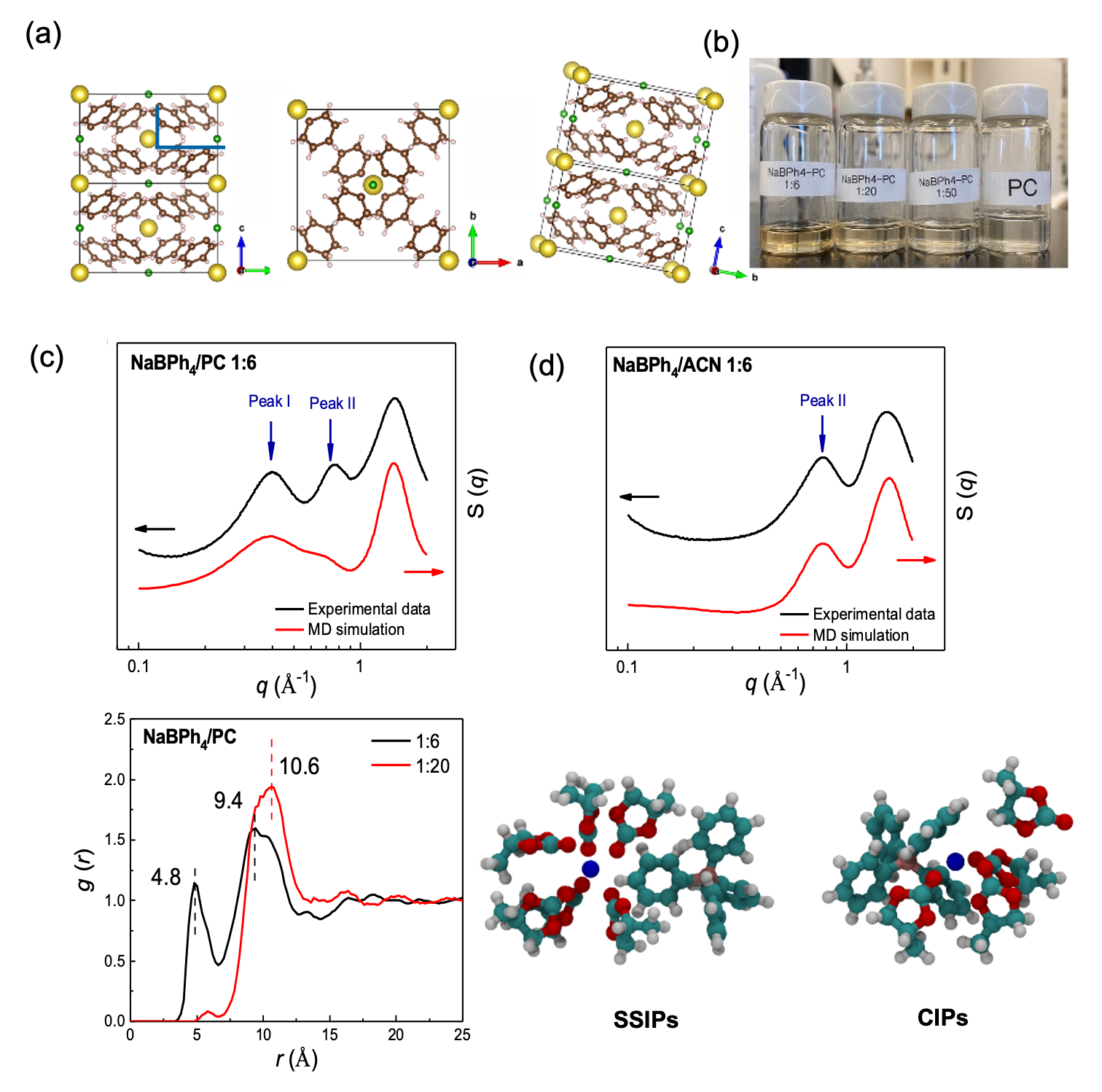
We compare the solvation phenomenon of sodium tetraphenylborate (NaBPh4) salt dissolved in organic solvents of propylene carbonate (PC), 1,2-dimethoxyethane (DME), acetonitrile (ACN) and tetrahydrofuran (THF) by SAXS/WAXS measurement and MD simulation.
Significance and Impact
Fluorine-free electrolytes have attracted great attention because of its low-cost and environmental friendliness. However, so far, little is known about the solution structures of these electrolytes. This work emphasizes the importance of global and local structural analysis of fluorine-free sodium electrolytes, which will provide valuable clues for understanding the structure-performance relationship of electrolytes.
Research Detail
- A unique structural feature is found in this salt-concentrated PC electrolyte by SAXS. Two characteristic peaks at 0.4 and 0.76 Å-1 emerged in concentrated NaBPh4/PC solution while only one peak at ~0.76 Å-1 can be found in NaBPh4 solutions using other solvents such as DME, ACN, and THF.
- This two-peak phenomenon is mainly caused by the short-range stacking between bulky BPh4- anions (clusters). The distances between clusters contribute to the unique peak at ~0.4 Å-1.
- Raman spectroscopy and MD simulations further confirm that there is a large population of CIPs in the concentrated NaBPh4/PC electrolyte.

