Scientific Achievement
Rechargeable Ca batteries have attracted little interest due to the extreme difficulty to perform reversible Ca electrodeposition. There is currently only one electrolyte, Ca(BH4)2 in THF, that exhibits reversible Ca deposition at room temperature. However, the underlying deposition mechanism and the interaction of Ca2+ ions with the surrounding molecules are still mostly unknown. Our research explores Ca deposition and speciation processes by using electrochemical and physical characterization techniques.
Significance and Impact
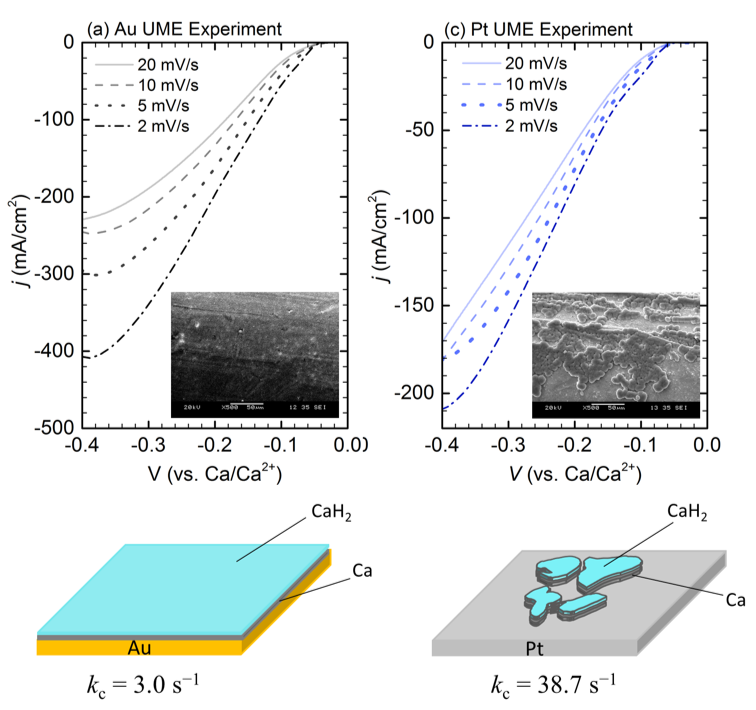
Our study suggests that Ca electrodeposition is governed by a chemical-electrochemical (CE) mechanism. The chemical step is related to the dehydrogenation of borohydride to produce adsorbed hydride ions that de-passivate the electrode surface, allowing deposition of Ca to proceed. Additionally, we found that Pt exhibits a higher chemical step rate (kc) than that at a Au electrode.
Research Details
- LSVs of Ca deposition at Au and Pt ultramicroelectrodes (UME) exhibit an inverse dependence between current density and scan rate, indicating the CE mechanism is operative.
- Mass spectrometry data suggests that the chemical step involves dehydrogenation of borohydride. Raman spectroscopy and mass spectrometry data show that Ca2+ ions are strongly coordinated to THF and weakly interacting with borohydride anions.
- We suggest that electrodeposited Ca interacts differently between Au and Pt electrodes due to differential rates of hydride formation on the two electrode surfaces.

