Scientific Achievement
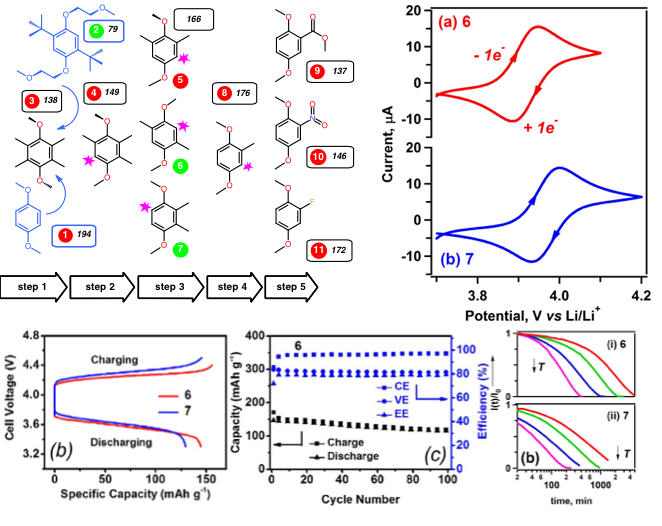
By systematically shedding molecular fragments of 2,5-di-tert-butyl-1,4-bis(2-methoxyethoxy)benzene that are considered important for radical cation steric stabilization, we discovered a minimalistic structure that retains long-term stability in its oxidized form and exhibits the intrinsic capacity of 161 mAh/g.
Significance and Impact
This study demonstrates the potential of the molecular pruning approach to discover low-weight redox active derivatives for electrochemical energy storage leading to materials with long-term stability and high intrinsic capacity.
Research Details
- 10 dimethoxybenzene derivatives have been designed and developed using a systematic pruning approach of shedding molecular fragments of 2,5-di-tert-butyl-1,4-bis(2-methoxyethoxy)benzene.
- In order to screen molecules with promising electrochemical performance, a stepwise work flow was conducted that consists of cyclic voltammetry, bulk electrolysis cell tests, and flow cell tests.
- Compound 6 and 7 show promising results during screening process and also afford much improved intrinsic capacity of 161 mAh/g.
- EPR analysis and DFT simulation reveal possible degradation pathways of the radical cations.

