Scientific Achievement
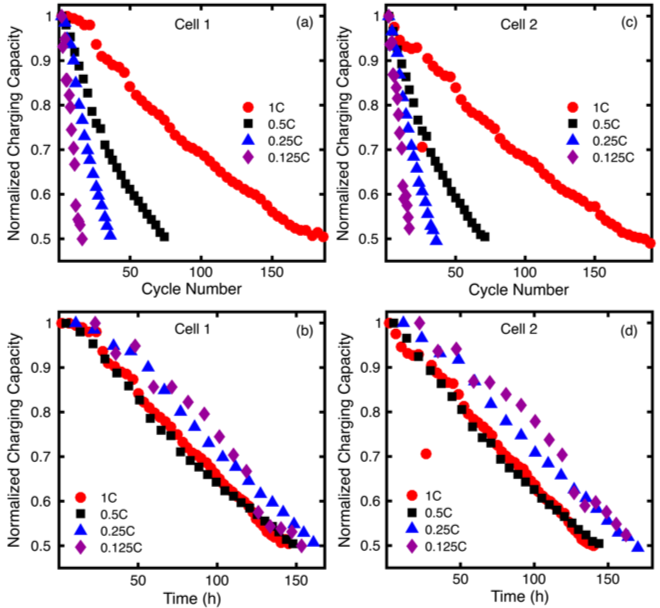
Under bulk electrolysis conditions, cycle time is a better measure of stability than cycle number and, for materials that are unstable in their charged state, the fractional capacity accessed is inversely related to cycle time until failure.
Significance and Impact
While standardized testing protocols for bulk electrolysis have yet to be agreed upon, consistency in data analysis can improve the comparison of materials-specific decay rates across the literature and consequently accelerate discovery science
Research Details
- We investigated the decay of a model compound, 2,5-di-tert-butyl-1,4-bis(2-methoxyethoxy)benzene (DBBB), under a variety of commonly reported bulk electrolysis cycling conditions
- Bulk electrolysis data is often reported as the capacity versus cycle number. However, at varying currents (C-rates), this “perceived stability” varies drastically.
- Cycling time, rather than cycle number, is a better representation of stability as relevant chemical decomposition reactions are time-dependent.
- Accessed capacity per cycle also impacts observed stability, due to the time- and concentration-dependence of decomposition processes.

