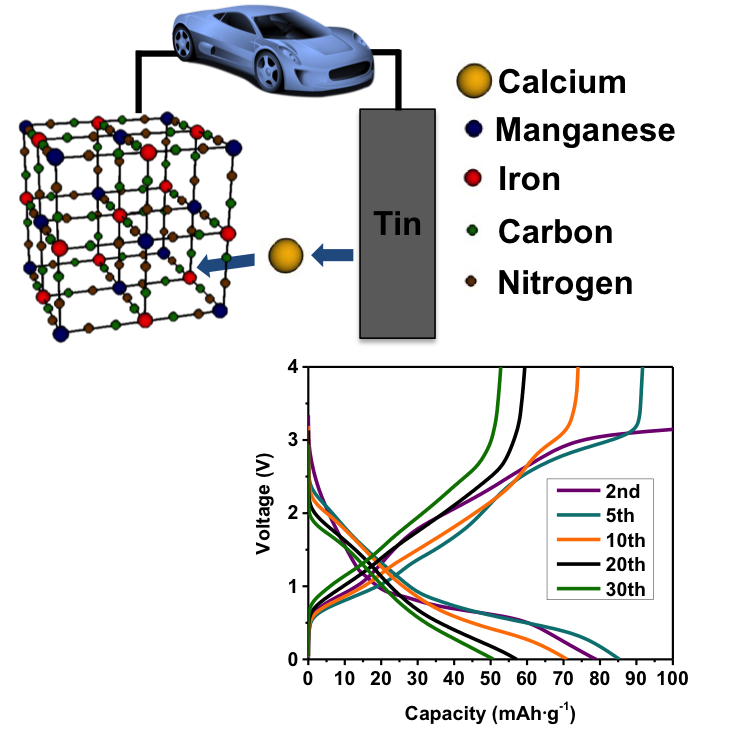
Scientific Achievement
We have demonstrated the first ever rechargeable battery utilizing calcium as the working ion.
Significance and Impact
This demonstrates the feasibility of using calcium as a working ion and furthermore gives the technical knowledge of how to do research on Ca-ion battery electrodes without the need for a functional calcium metal anode.
Research Details
- Demonstrated the use of a capacitive counter electrode that can be used with carbonate based electrolytes
- Proved that the Prussian blue analogue cathode material can intercalate Ca via multiple characterization techniques
- Calcium content increases and decreases during cycling (energy dispersive X-ray spectroscopy)
- Manganese oxidation state changes as expected with cycling (X-ray absorption near edge structure)
- Reversible structural changes that indicate expansion and contraction during cycling (X-ray diffraction)
Work performed at Argonne National Laboratory (JCESR managing partner) by Lipson, A. L., Pan, B., Lapidus, S. H., Liao, C., Vaughey, J. T. and Ingram, B. J., “Rechargeable Ca-Ion Batteries: A New Energy Storage System,” Chem. Mater. 2015.

