Scientific Achievement
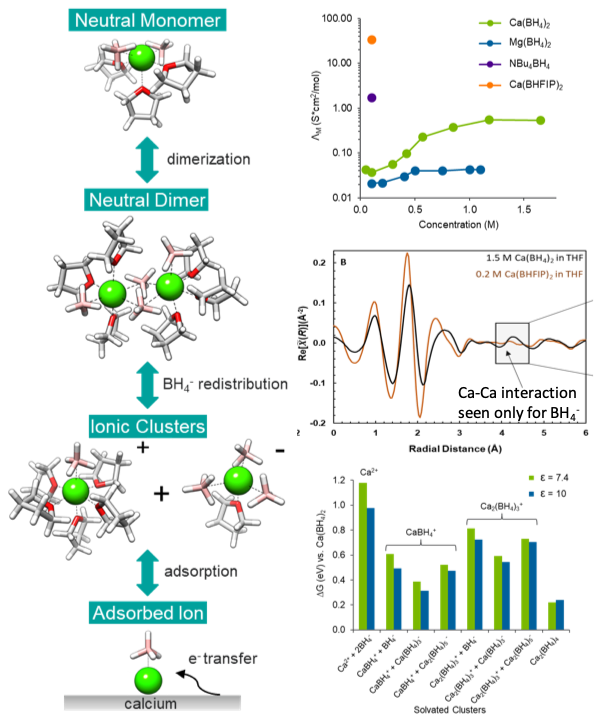
Calcium borohydride in tetrahydrofuran is effective at reversible multivalent metal deposition where magnesium borohydride is not because the more polarizable Ca2+ cation enables greater coordination flexibility resulting in enhanced formation of CaBH4+ produced via multimer intermediates.
Significance and Impact
A new model for MV dication solvation is demonstrated for strongly associated salts in low permittivity solvents whereby increased ionicity with salt concentration results from neutral multimer formation followed by anion exchange, representing a lower energy path for ionic cluster formation. A coordination-activity series for alkaline earth dications emerges to support electrolyte design for electrochemically stable ether solvents.
Research Details
Identification of a unique trend in electrolyte ionicity
In situ X-ray absorption demonstration of Ca2+ multimer species
Computational determination of favorable ionic clusters containing associated Ca2+ and BH4-
Solvate single crystal assessment of multimer formation tendency across the alkaline earth metal series (Mg2+, Ca2+, Sr2+, Ba2+) demonstrating a key transition between Mg2+ and Ca2+

