Scientific Achievement (18pt Arial, Bold)
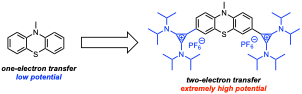
High redox potential, two-electron organic catholytes for non-aqueous redox flow batteries were developed by appending diaminocyclopropenium (DAC) substituents to phenazine and phenothiazine cores.
Significance and Impact (18pt Arial, Bold)
The incorporation of DAC substituents has a dual effect on these systems. The DAC groups increase the redox potential of both couples by ~300 mV while simultaneously rendering the second oxidation (which occurs at 1.20 V vs Fc/Fc+ in the phenothiazine derivative) reversible.
Research Details (18pt Arial, Bold)
- The electron-withdrawing nature of the DAC unit is responsible for the increase in redox potential, while the DAC substituents stabilize oxidized forms of the molecules through resonance delocalization of charge and unpaired spin density.
- These new catholytes were deployed in two-electron redox flow batteries that exhibit voltages of up to 2.0 V and no detectable crossover over 250 cycles.

