Scientific Achievement
Our results shed light on the interfacial electrochemistry of silicon anodes for Lithium-ion batteries (LiBs), providing important mechanistic insight into nanometer scale phenomena and how these influence battery performance.
Significance and Impact
This work motivates further fundamental model-system-based studies of LIB components, of both experimental and computational as well as theoretical nature, and we anticipate that such studies can aid in achieving our goals of improved battery materials.
Research Details
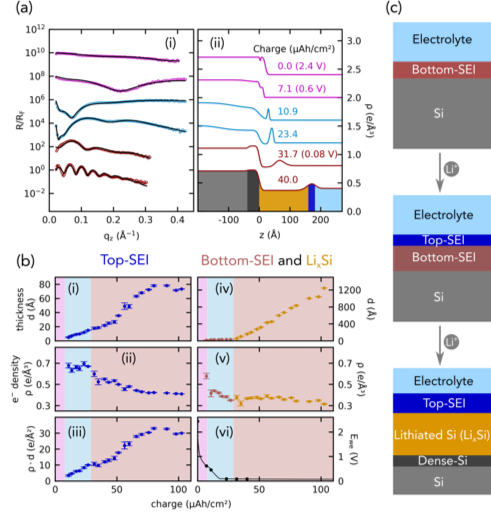
- Model battery anode consisting of a native oxide terminated single crystalline Si wafer
- Observed growth of two inorganic SEI layers, LixSiOy (Lithiation of electrode-adjacent) and LiF (electrolyte-adjacent)
- Crystalline Si (c-Si) is a layer-by-layer, reaction-limited, two-phase process
- Delithiation of LixSi and the lithiation of amorphous Si (a-Si) are reaction-limited, single-phase processes
- Unraveled the influences of current density and the Si crystallographic orientation

