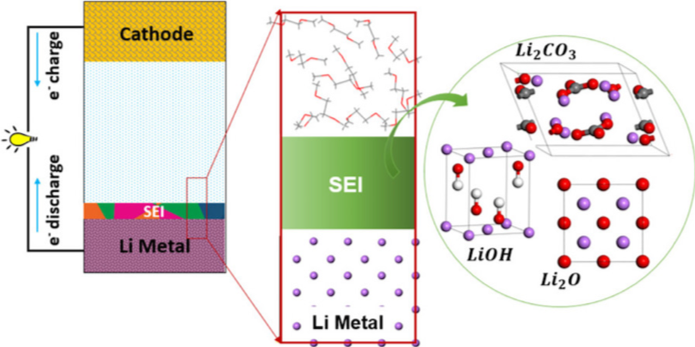
Scientific Achievement
We uncovered the effects of nanometer-sized lithium oxide, lithium hydroxide, and lithium carbonate as surface passivation layers on the interfacial reactivity of Li-metal anodes.
Significance and Impact
Our results are an important step towards predicting the chemical, structural, and topographical heterogeneity of solid electrolyte interphase layers arising from a multitude of interfacial constituents.
Research Details
- Solution decomposition experiments were performed in pure solvent (dimethoxyethane) and then with the addition of 1 M of Li-bis(trifluoromethanesulfonyl)-imide.
- When lithium oxide was the major component on the altered surface, lithium fluoride phases formed. The presence of a dominant hydroxide layer resulted in enhanced decomposition processes involving sulfur.
- Our observations can be explained based on the calculated quantities of electronic charge transfer found for each of the passivating films.

