Scientific Achievement
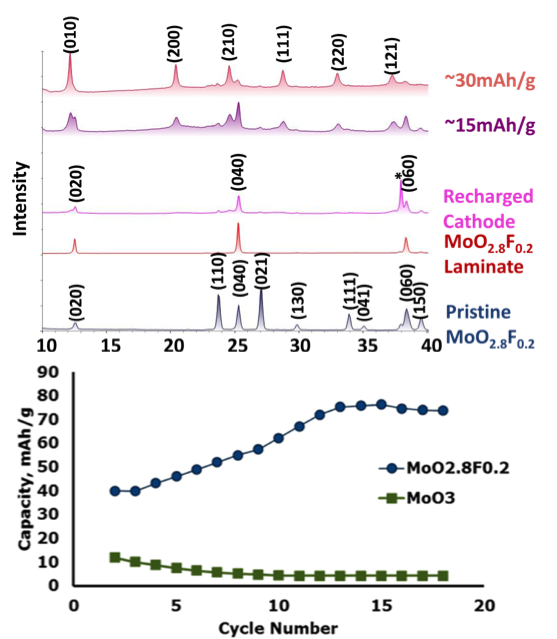
Magnesium was reversibly intercalated at room temperature into an oxyfluoride cathode without the co-intercalation of electrolytes or protons and without the formation of unwanted side-products that commonly plague oxide cathodes.
Significance and Impact
The cathode promises improved battery cyclability and capacity fade while paving the way for high voltage multivalent cathodes that work analogously to lithium-ion.
Research Details
- Electrochemical testing of the material was supported by powder X-ray diffraction, Nuclear Magnetic Resonance spectroscopy, and first-principles computational modeling.
- Differences in the capacity of the Molybdenum oxide and the oxyfluoride suggest that electrical conductivity of the cathode plays a strong roll in the facile intercalation of Mg.
Work performed at Northwestern University (JCESR partner), Lawrence Berkley National Laboratory (JCESR partner), and Argonne National Laboratory (JCESR managing partner) by J. Incorvati, L. Wan, B. Key, D. Zhou, C. Liao, L. Fuoco, M. Holland, H. Wang, D. Prendergast, K. Poeppelmeier and J. Vaughey, Chemistry of Materials, 2015

