Scientific Achievement
A new nonaqueous all-organic redox flow chemistry was developed with a cell voltage of ~2.4V, energy efficiency of >70%, and operational current density of >10mA/cm2.
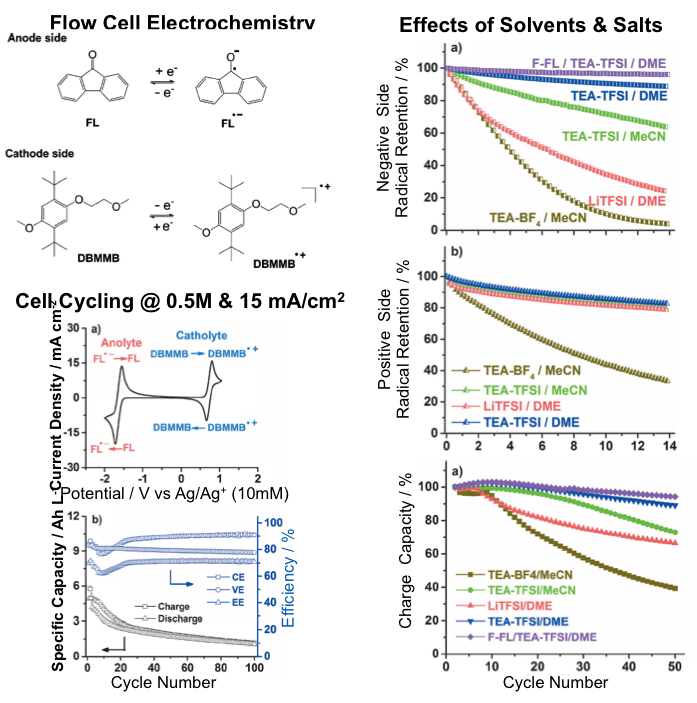
Electron spin resonance (ESR) study reveals that the chemical stability of both charged organic radical species dictates the cycling stability of flow cells and is highly dependent on the supporting solvents and salts.
Significance and Impact
The choice of solvents and salts and the structural tailoring of organic redox materials can be used to improve the chemical stability of redox species, thus cycling stability of flow cells.
Research Details
- Cell electrochemistry of FL and DBMMB was studied by using both cyclic voltammetry and real flow cell configurations.
- Effects of solvents and salts on flow cell cycling stability were investigated systematically. Cell performance degradation mechanism was explored using ESR.
Work performed at Pacific Northwest National Laboratory (JCESR partner) and Argonne National Laboratory (JCESR managing partner) by X. Wei, W. Xu, J. Huang, L. Zhang, E. Walter, C. Lawrence, M. Vijayakumar, W. A. Henderson, T. Liu, L. Cosimbescu, B. Li, V. Sprenkle and W. Wang, Angew. Chem. Int. Ed. 2015,

