Scientific Achievement
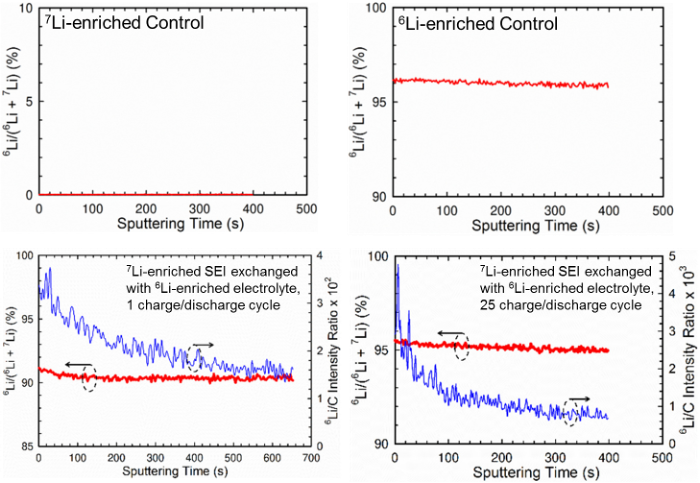
By using Li isotopic labelling of SEIs and electrolytes followed by time-of-flight secondary-ion mass spectroscopy and solid-state NMR analyses, we found that the majority of Li+ “immobilized” in the chemical ingredients were exchanged after 1 SEI formation cycle. Ion exchange by diffusion based on concentration gradient without applied potential also occurred simultaneously.
Significance and Impact
SEI is the most important and yet the least understood component in lithium-ion batteries, despite extensive studies in the past two decades. Few questions, such as the chemical form of and degree of mobility of Li+ in the interphase and the fraction of Li + that is permanently immobilized in the SEI, remain unanswered. The results of this study contributed to the foundation for both understanding and designing better SEIs for future battery chemistries.
Research Details
- An SEI on graphite anode half-cell was first formed using 7Li-enriched electrolyte and foil, after which, the solvent-rinsed delithiated graphite anode was reassembled and cycled again using the same number of cycles as the original SEI but with 6Li-enriched electrolyte and foil.
- Time-of-flight secondary-ion mass spectroscopy and solid-state NMR were employed to quantify amount of isotope exchange.
- Lithiation/delithiation capacity and capacity loss as a function of cycle number before and after isotope exchange were monitored.

