Scientific Achievement
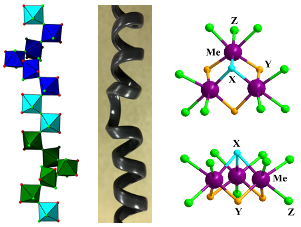
The compound families (i) (Rb/NH4/Tl)MoO2F3, (ii) K5[Mo3O4F9]·3H2O, K5[Mo3O4F9]·2H2O, K16[Mo3O4F9]2[TiF6]3·2H2O, and (iii) Δ,Λ-[Cu(bpy)2(H2O)]2[(Ti/Zr/Hf)F6]2 were synthesized by hydro(solvo)thermal methods, and their atomic structures were solved with single-crystal X-ray diffraction and solid-state 19F NMR spectroscopy. These materials exhibit unique features such as (i) periodic tendril perversion (a reversal in helical chirality) along a 1D chain, (ii) metal–metal bonded heteroanionic clusters, or (iii) noncentrosymmetric crystallization of racemic mixtures.
Significance and Impact
The new crystallization motifs in these structures open new avenues for the discovery of ion conducting materials. Most of these new compounds exhibit anion dynamics that may be related to the concept of the paddlewheel conduction mechanism.
Research Details
Exploratory hydrothermal synthesis in the early transition metal oxyfluoride systems in search of new Mg cathode materials (e.g. MoO2.8F0.2) resulted in the synthesis of a dozen compounds.
The crystal structures of these compounds were solved via single crystal X-ray diffraction, revealing new structural phenomena.
Recent work aims to characterize the new oxyfluoride materials using 19F NMR, which can identify order/disorder and materials with anion dynamics that are candidates for Mg conductivity.

