Scientific Achievement
Redox-active oligomer molecules can become more (100x) stable when they are fully charged compared to their partially charged states. This is the first time this effect was observed in chemistry.
Significance and Impact
The stability of redoxmers containing multiple redox units is relatively uncharted territory in flow battery electrolyte fluids. Our observations suggest that the JCESR strategy of using multiply charged macromolecular redoxmers in combination with nanoporous polymer membranes will not be derailed by the increasing destabilization of such molecules in electrolyte. Coupled with sufficient molecular design, stabilization is achieved.
Research Details
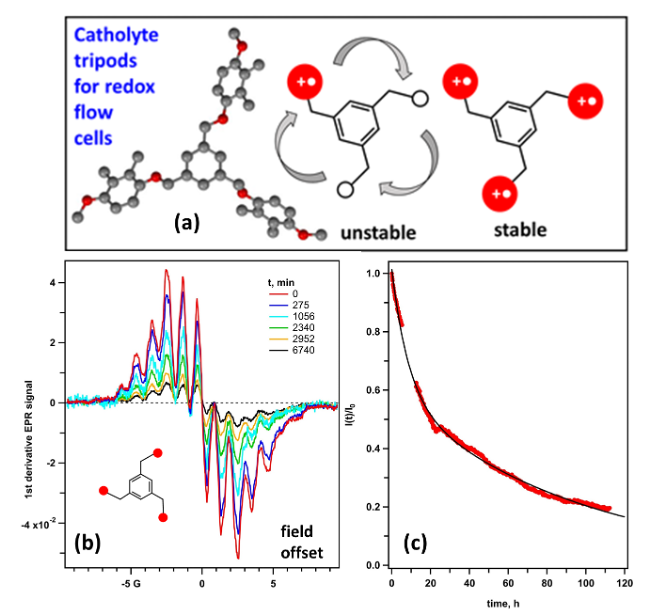
Three-legged (tripodal) redoxmers are propeller like macromolecules whose “blades” are redox-active units that store positive charge in flow batteries (panel a of the figure)
- Two tripods were synthesized, one capable of only being singly-charged and one capable of being triply charged
- When the tripod is charged, the “blades” interact with each other. Does this interaction make the molecules less stable or more stable?
- Using electron spin resonance, product analyses, and molecular dynamics we show that, counter intuitively, in some cases the fully charged tripods are more stable than the partly charged ones. One leg bad, three legs good!

