Scientific Achievement
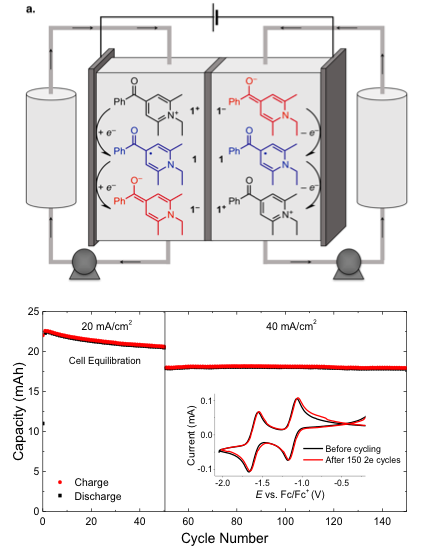
High energy density organic redox flow batteries can only be realized when stable light-weight organic redox systems are developed, multi-electron redox molecules in combination with light-weight supporting electrolytes pave the way to this goal.
Significance and Impact
Potassium hexafluorophosphate (KPF6) has been identified to dramatically lower the equivalent weight of a recently developed organic anolyte system, while supporting flow cell cycling through two redox events at low potentials without detectable degradation of the redox material.
Research Details
- Evaluation of various alkali electrolyte salts led to the selection of KPF6 as a prime candidate in order to reduce the equivalent weight of the redox system and meet techno-economic targets.
- Stable two electron cycling of a promising pyridinium based anolyte has been demonstrated for 150 cycles with the use of KPF6 as the supporting electrolyte.

