Scientific Achievement
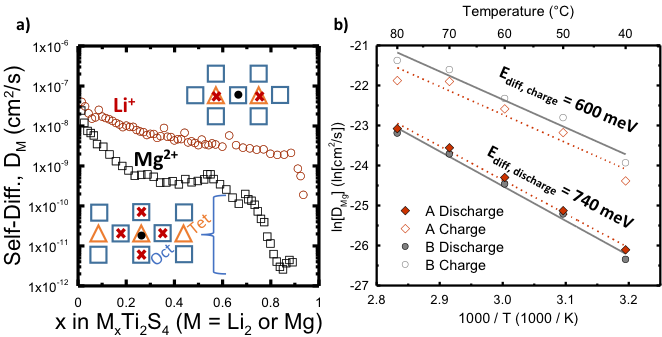
We showed why the positive electrode material, MgxTi2S4, does not work well at room temperature, unlike Li2xTi2S4, which works very well. Furthermore, we confirmed that theoretical diffusion calculations predict accurate results at low x values.
Significance and Impact
Since diffusion rates can be accurately predicted, the search for new Mg positive electrode materials would be more efficient if diffusion coefficients were calculated for candidate materials before investing time in making and testing them. A diffusion barrier of less than about 500 meV will be required for a useful, dense Mg positive electrode material.
Research Details
- Diffusion coefficients were measured using a novel Galvanostatic Alternating Pulse method & pressed pellets.
- The decrease in DMg above about x = 0.6 could be due to either (or both) an increase in Mg2+ – Mg2+ interactions within the lattice or to Mg tetrahedral site occupation.
Work performed at the University of Waterloo (JCESR collaborator) and Argonne National Laboratory (JCESR managing partner) by P. Bonnick, X. Sun, K. Lau, C. Liao, and L.F. Nazar, J. Phys. Chem. Lett., 2017, 8, 2253-2257

