Scientific Achievement
A direct connection between molecular structure and liquid viscosity was made, and the latter was explained based on the former feature.
Significance and Impact
The quantitative connection between molecular structure and liquid transport properties such as viscosity makes the accurate prediction of the liquid property possible, which will speed up the design process of new electrolyte systems.
Research Details
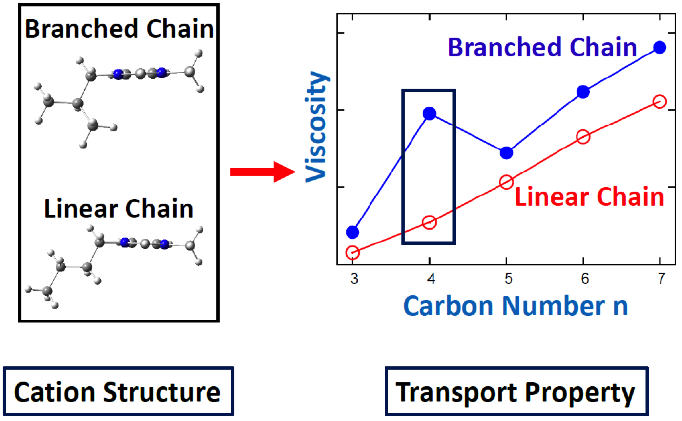
- Using classical molecular dynamics (MD) simulations, the experimental trend in viscosity of two series of ionic liquids were reproduced.
- Detailed analysis based on MD simulations reveals that the liquid viscosity is related to the packing structure in the liquid phase, which in turn is dictated by the molecular structure.
- The abnormally high viscosity in one branched chain IL was found to be a result of the specific side chain length and molecular structure.
Work performed at University of Notre Dame (JCESR collaborator) and Texas Tech University by Y. Zhang, L. Xue, F. Khabaz, R. Doerfler, E.L. Quitevis, R. Khare, and E.J. Maginn. J. Phys. Chem. B, 2015, 119, 14934-14944

