Scientific Achievement
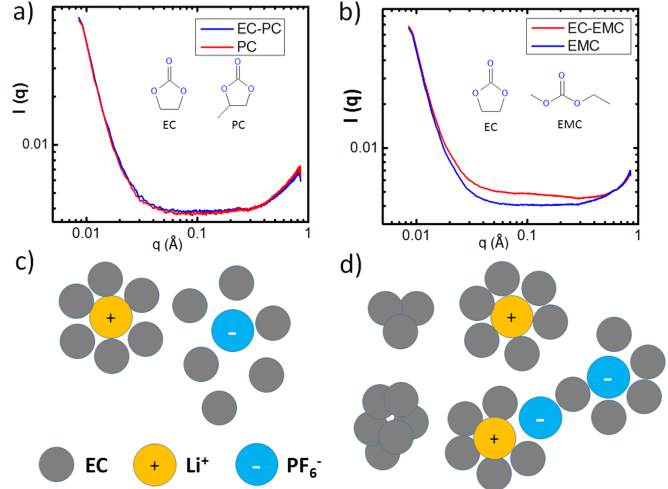
We report, for the first time, the observation of EC nanoclusters in both EC/EMC solvent mixture and the LiPF6/EC/EMC electrolyte through Small Angle X-ray Scattering (SAXS) studies. We detect and estimate the size of LiPF6 ion pair in these carbonate-based electrolytes.
Significance and Impact
These unexpected findings of aggregate structures reveal the knowledge gap between the microscopic structures to macroscopic properties of battery electrolytes. The SAXS technique is a useful tool for studying and understanding non-ideal behaviors of electrolytes.
Research Details
- EC forms clusters of ∼1 nm in linear carbonate (EMC or DEC), while no clusters is formed in cyclic carbonate, PC.
- Structure and size of EC clusters remains the same even in the presence of high concentration LiPF6 (1M), e.g. in LiPF6/EC/EMC, LiPF6/EC/DEC.
- LiPF6 contact ion pair is only observed in the EC/EMC electrolyte, not in EC/PC mixtures at the concentration studied due to the differences in dielectric constant between EMC and PC; the size of ion pair in EC/EMC is measured to be ∼0.6 nm.

