Scientific Achievement
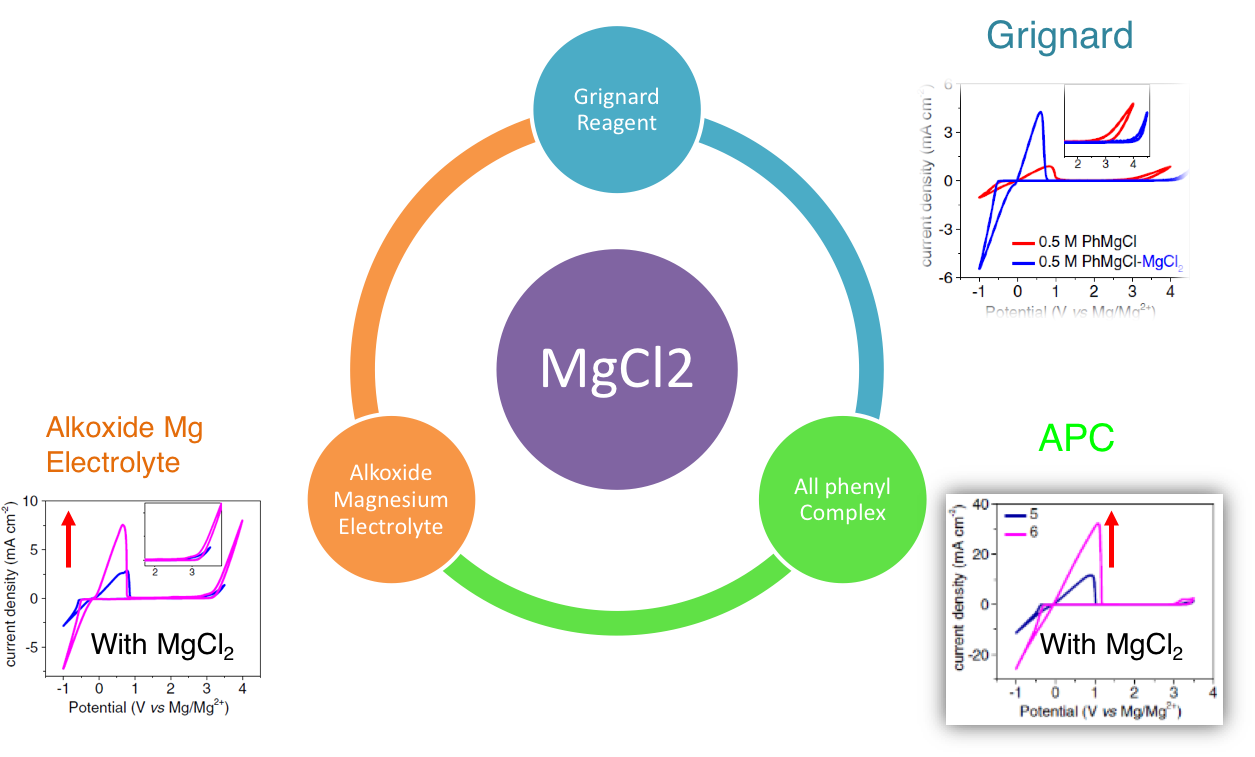
The effect of MgCl2 on a series of chloride containing magnesium electrolytes has been experimentally investigated. The incorporation of MgCl2 into these electrolytes results in the significant improvement in all aspects of electrochemical properties.
Significance and Impact
MgCl2 has also proven to be a powerful reagent to replace Lewis acid and improve the performance of well-established strong Lewis acid derived magnesium electrolytes such as the “all-phenyl” complex (APC) and alkoxide-based magnesium electrolytes. Our extensive experiments suggest that MgCl2 should be the key species for all chloride containing electrolytes for non-aqueous rechargeable magnesium-ion batteries.
Research Details
- Addition of benign salt MgCl2 into Grignard reagent, APC and other alkoxide based Mg electrolytes enables the electrolyte to show both deposition and high anodic stability . The excellent battery cycling performance of these X-MgCl2 (X is the original Mg electrolytes) electrolytes using Chevrel phase Mo6S8 further demonstrates the great potential of our electrolyte for rechargeable magnesium-ion batteries.
Work performed at Argonne National Laboratory (JCESR managing partner) by B. Pan, J. Huang, N. Sa, S. M. Brombosz, J. T. Vaughey, L. Zhang, A. K. Burrell, Z. Zhang, and C. Liao, Journal of The Electrochemical Society 2016, 163, A1672-A1677.

