Scientific Achievement
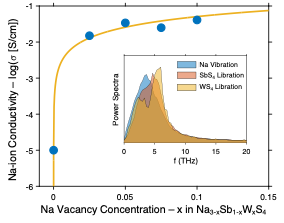
The atomic-scale mechanisms that underlie the exceptionally high ionic conductivity of Na3-xSb1-xWxS4 are elucidated. The conductivity is well explained by a combination of vacancy-related effects and a strong overlap of cation vibrational modes with anion librations.
Significance and Impact
Recently, the solid electrolyte, Na3−xSb1−xWxS4, was reported to exhibit a conductivity for Na-ions exceeding that of all lithium and sodium solid conductors. Notably, this compound’s crystal structure contains complex anions—specifically, tetrahedral SbS4 and WS4. Prior studies on related solid electrolytes have argued that reorientations of the complex anions facilitate cation mobility through the so-called paddlewheel effect. The present study investigates the extent to which paddlewheel dynamics and other factors influence ionic conductivity in this important material.
Research Details
- ab initio molecular dynamics was used to model the atomic-scale processes associated with ion migration
- Correlations between the dynamics of Na-ions and that of the surrounding complex-anion “cage” were characterized to identify whether paddlewheel dynamics contribute to the high cation conductivity
- Limited evidence was found for contributions to Na migration from SbS4 and WS4 reorientations

