Scientific Achievement
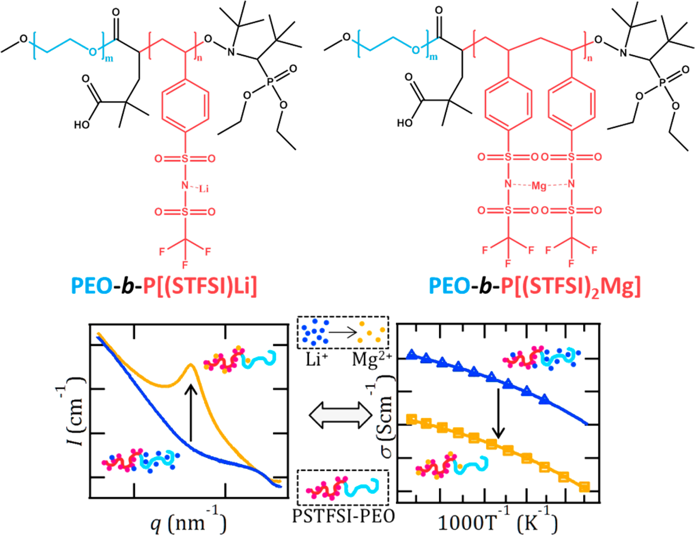
Ion dissociation and block copolymer thermodynamics are intimately coupled: ion dissociation in lithiated systems suppresses microphase separation.
Significance and Impact
A linear relationship between the charge-concentration-related VTF parameter and the parameter quantifying the enthalpic contribution to χ, indicates ion dissociation and block copolymer thermodynamics are intimately coupled.
Research Details
- The melt morphology of the single-ion conducting block copolymers is studied using temperature-dependent X-ray scattering and use the mean-field theory of Leibler to extract the effective Flory−Huggins interaction parameter (χ) for PEO/P[(STFSI)Li] and PEO/P[(STFSI)2Mg] from the X-ray scattering data.
- The temperature dependence of ionic conductivity was measured, and through analysis using the Vogel−Tamman−Fulcher (VTF) relation, demonstrate that ion dissociation is significantly lower for all PEO−P[(STFSI)2Mg] samples when compared to their PEO−P[(STFSI)Li] counterparts.
DOI: 10.1021/acs.macromol.6b01886

