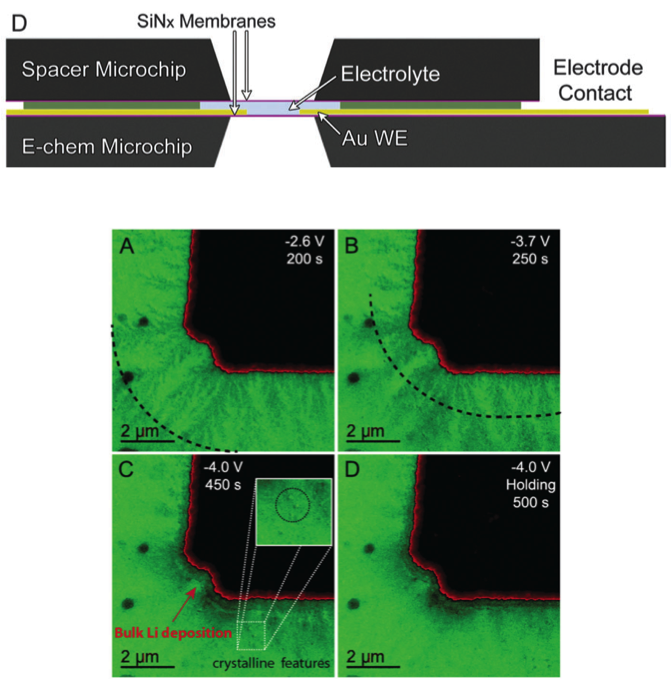
(Bottom) How battery fading evolves through dendrite formation during battery operation. Top: The wave front (dotted line) corresponds to amorphization of the SEI dendrites by lithium deposition. Bottom: The Li deposition/SEI layer formation. The inset spotlights of crystalline features.
Scientific Achievement
Demonstrated that formation of solid electrolyte interphase (SEI) as dendrites occurs before lithium electrodeposition on the gold electrode and remains after lithium electrodissolution, changing our view of dendrite formation and the safety of lithium-ion batteries.
Significance and Impact
- Provides in-situ, real-time information on the dendrite growth mechanisms and kinetics during battery operation.
- Established a liquid cell based platform to study real time, in-situ molecular interactions inside batteries. Typical studies are post-mortem, after charge/discharge cycles or via computational modeling of particular battery system.
Research Details
- Characterized dendrite formation at the gold electrode submersed in a LiPF6 in EC:DMC electrolyte at high spatial resolution using a new in-situ liquid electrochemical cell placed in a transmission electron microscope
- Crystalline particles were formed during Li deposition. Future studies may show if these particles are Li metal. If so, a conduction pathway may be formed through the SEI that facilitates the growth of dendrites
Robert Sacci et al. Chemical Communications, 2014.

