Scientific Achievement
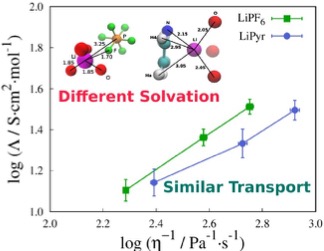
MD simulations show that the ionic dynamics of electrolyte solutions can be similar even though the Li+ solvation in each solution is different.
Significance and Impact
Two electrolytes with similar transport behavior but different solvation structure may have different reaction intermediate solubility and distinct reaction mechanisms, which provide insights for experimental design and analysis addressing electrolyte impact over Li-O2 devices.
Research Details
- LiPF6 and LiPyr in DMSO solutions were studied using classical molecular dynamics simulations.
- The calculated shear viscosity, diffusion coefficient and ionic conductivity were found to be similar in the two electrolytes regardless of the anion type and shape
- The structural arrangement in the two electrolytes presents completely different scenario. In LiPF6/DMSO solution, Li+ is fully solvated by DMSO; whereas in LiPyr/DMSO solution, Li+ is partially shared between DMSO and Pyr anion.

