Scientific Achievement
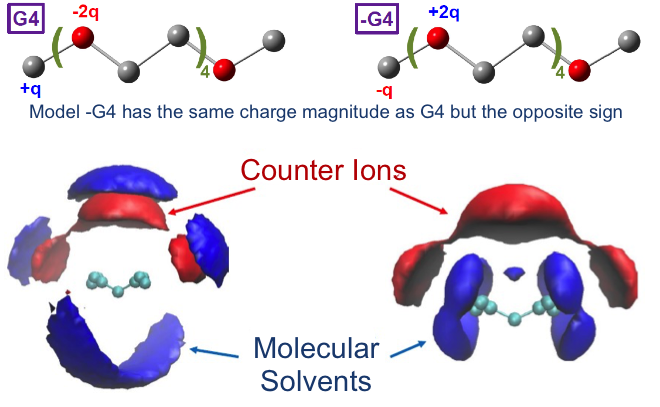
Charge localization of the solvent molecules affects the liquid phase structure and transport properties in electrolyte solutions.
Significance and Impact
Classical molecular dynamics simulations revealed that solvent molecules with localized negative charges tend to coordinate strongly with cations in the solution and decreases their dynamics. Therefore, in order to increase cation (e.g. Li+, Mg2+) dynamics, a solvent with localized positive charges is preferred.
Research Details
- Liquid structure and dynamic properties of electrolyte model systems were studied at different concentrations using both classical molecular dynamics simulation and experimental techniques.
- Anion dynamics were improved as more molecular solvents were added to the solution due to strong coordination between cations and solvents
Work performed at University of Notre Dame (JCESR Partner) by A. Sharma, Y. Zhang, T. Gohndrone, S. Oh, J.F. Brennecke, M.J. McCready, and E.J. Maginn. Chem. Engineer. Sci., 2016

