Scientific Achievement
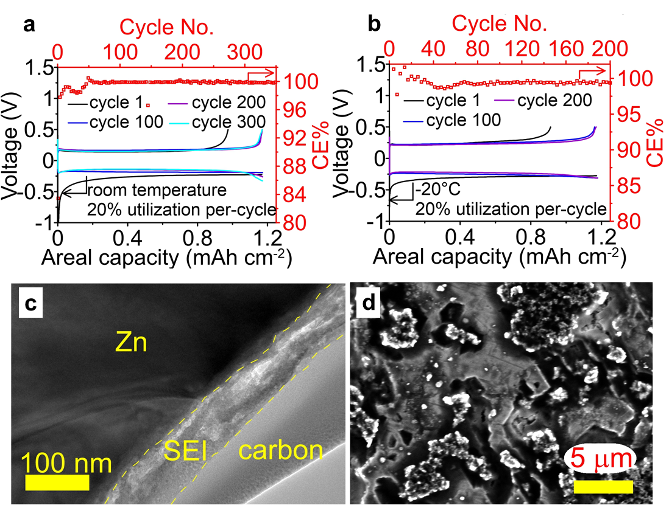
A liquefied gas electrolyte was developed for the 1st time for a bivalent chemistry. It displays an excellent Zn conductivity (>3.4 mS cm-1) across a broad temperature range (-60 to +20 °C), enables highly reversible Zn cycling with no evidence of shorting behavior at both room temperature and -20 °C for over 200 cycles (>400 h) with a high Coulombic efficiency (CE > 99%) at high utilization (20% Zn per cycle) for Zn plating/stripping at both room temperature and -20°C.
Significance and Impact
This is a promising new direction to achieve a highly reversible Zn metal anode supporting high energy density rechargeable Zn metal batteries across a wide temperature range for Zn metal anode and Zn||Na2V6O16·1.63H2O full cells.
Research Details
- The effect of this liquefied gas electrolyte on the Zn interphasial structure and chemistries was investigated using sputtering XPS, TEM-EDX and DFT calculations.
Combining sputtering XPS, TEM, XRF and DFT calculation, we revealed the potential capacity resource in this liquefied gas electrolyte when paired with Na2V6O16·1.63H2O as cathode

