Scientific Achievement
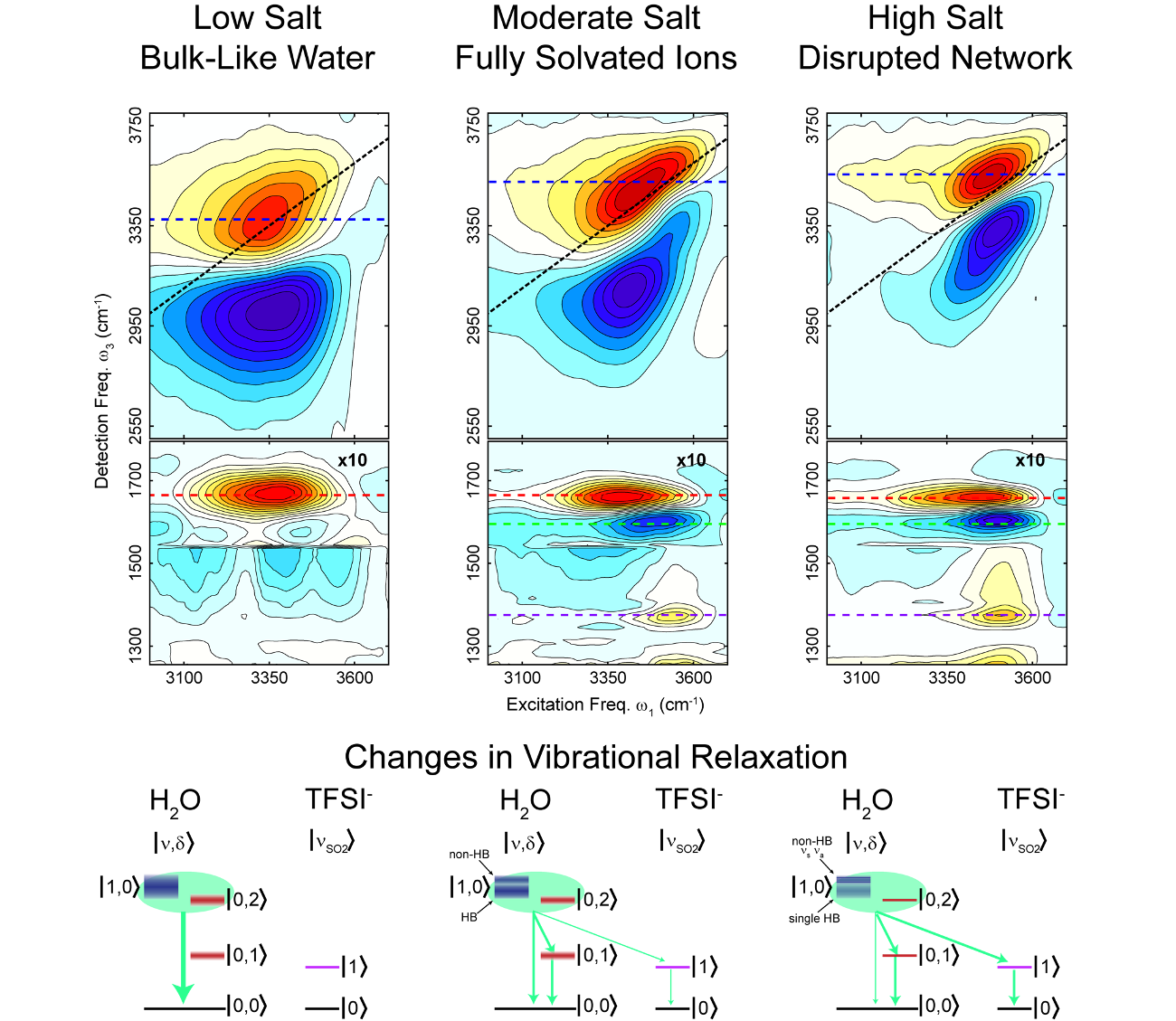
Using ultrafast infrared spectroscopy and molecular dynamics simulations we uncover details of the molecular-scale structure of super-concentrated aqueous electrolytes.
Significance and Impact
We observe the changes in the water morphology in electrolytes from the dilute to the superconcentrated regime. In the superconcentrated solutions we find the water network is completely disrupted, and the vibrational relaxation properties are distinct from the pure liquid. These structural changes have significant impact on the ion mobility in batteries utilizing these electrolytes.
Research Details
- Combining IR spectroscopy and MD simulations we elucidate the changes in the water hydrogen bonding network in battery-relevant aqueous electrolytes.
- We reveal the complete disruption of the water network at high salt concentration, disproving prevalent theories for the primary mechanism of ion transport in these solutions.
- By tracking the changes as a function of ionic concentration we reveal a crossover from the bulk-like water to the ‘water-in-salt’ electrolyte regime, elucidating long-standing questions about the properties of water.

