Scientific Achievement
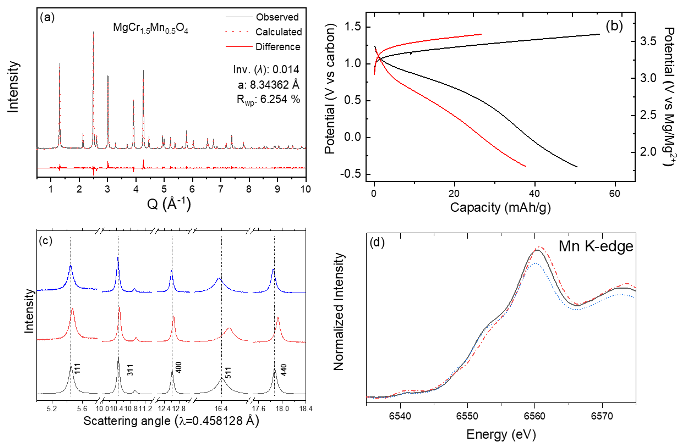
Inversion-free spinel MgCr1.5Mn0.5O4 was successfully synthesized. The tailored spinel showed reversible (de)intercalation of Mg2+ at high redox potentials. It was found that the overpotentials and, thus, overall hysteresis was reduced when the inversion ratio in the spinel lattice was minimized. The experimental evidence emphasizes the influence of structural defects, in this case inversion, on electrochemical Mg2+ activity and provides a design rule toward a building functional Mg cathode for a high-energy Mg-ion battery.
Significance and Impact
Our findings enhance the understanding of Mg2+ transport within spinel oxide frameworks and provide conclusive evidence for bulk Mg migration in oxide lattices at high redox potentials with minimized electrochemical hysteresis.
Research Details (18pt Arial, Bold)
- An inversion-free spinel, MgCr1.5Mn0.5O4, was synthesized by exploiting the different intrinsic crystal field stabilization of redox-active Cr and Mn in the form of a solid-solution.
- Multimodal characterization by X-ray diffraction, X-ray absorption spectroscopy, and solid-state NMR indicated that structural, compositional, and redox changes were consistent with the observed electrochemical Mg2+ activity in the spinel oxide.

