Scientific Achievement
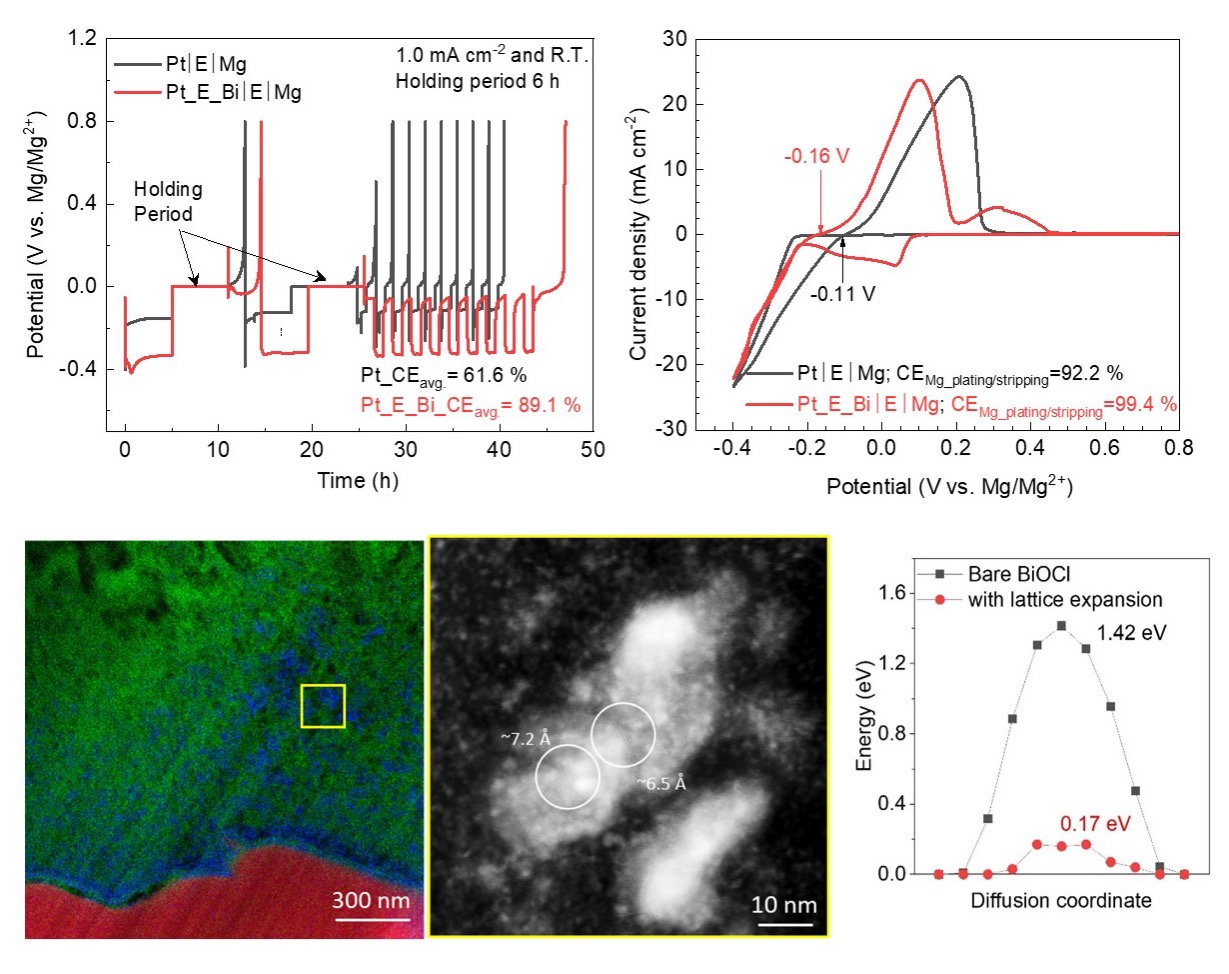
The dynamics and instability of the Mg/electrolyte interface over extended time were revealed and an artificial interfacial layer with fast ion transfer kinetics was developed to suppress side reactions and thus improve electrochemical stability.
Significance and Impact
An electrochemically activated artificial layer enables robust electrochemical cycling under practical testing conditions. The major component of the artificial layer, a lattice-expanded oxychloride, presents a much lower energy barrier for the diffusion of active cationic species, resulting in ultra-fast charge transfer kinetics.
Research Details
- Galvanostatic cycling of a Bi nanolayer in a MgCl2-based electrolyte resulted in an artificial interphase layer on a Mg anode that improves the plating/stripping efficiency and sustainability.
- High-resolution spectroscopic and microscopic analysis revealed that the resulting film consisted of lattice-expanded fibrous BiOCl spatially well-dispersed in a MgOCl/F/S matrix, which supported efficient the transport of active cationic species (MgCl+) as also demonstrated by DFT calculations.

