Scientific Achievement
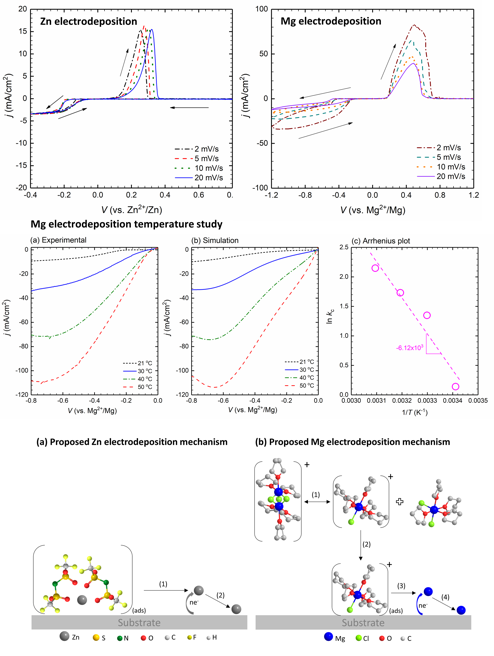
The development of nonaqeuous electrolytes enabling reversible and efficient deposition/stripping of multivalent metals has been hindered because of the complexity of electrolyte properties and behaviors. Different cations exhibit different deposition efficacy. Our research explores Zn and Mg deposition mechanisms and explains differences between the two cations.
Significance and Impact
Our research shows that Mg and Zn electrodeposit by two fundamentally different mechanisms. Mg requires a chemical step in addition to an electron transfer step, while Zn just requires an electron transfer step. This result suggests that facile Mg deposition may be reached at higher temperatures whereas Zn electrodeposition may be enhanced by increasing electron-transfer rate constant, likely through additives.
Research Details
- CVs of Zn deposition at a ultramicroelectrode (UME) exhibit an independent relationship between current density and scan rate indicating a simple two-electron reduction mechanism.
- CVs of Mg deposition at a UME show an inverse dependence between scan rate and current density suggesting a more complicated chemical-electrochemical process.
- By coupling electrochemistry with COMSOL simulation, our study provides kinetic parameters and insights central to the Zn and Mg electrodeposition/dissolution processes.

