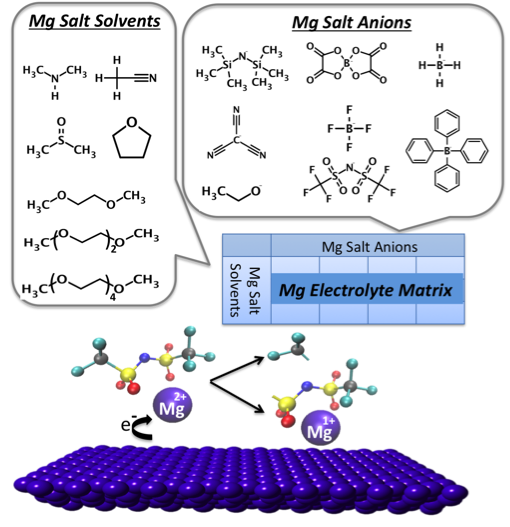
(Bottom) The TFSI- decomposition mechanism triggered by partial reduction of Mg2+ –>Mg+. This mechanism consumes anion, disrupts the deposition of Mg0 on the anode and may deposit anion fragments on the anode.
Scientific Achievement
- Simulations of a matrix of Mg salt and solvent combinations revealed a strong tendency to ion pair formation. Close association of the salt anion and cation within the first solvation shell, even at modest concentrations.
- At a certain potential in the charge/discharge cycle, the ion pair undergoes partial reduction of the Mg cation, Mg2+ –> Mg+. This disrupts full reduction of Mg2+ –> Mg0 that is required for deposition of metallic Mg on the anode.
- Partial reduction of Mg2+ –> Mg+ triggers rapid decomposition of the paired anion in a downhill exothermic reaction that consumes the salt and may deposit anion fragments on the anode. This is a catastrophic failure mode for the battery.
Significance and Impact
We find the TFSI– anion to be highly susceptible to decomposition triggered by partial reduction of Mg2+ –> Mg+, in contrast to other ions such as BH4– and BF4–, which are shown to be stable in the presence of a transient, ion-paired Mg+ ion. This may provide an explanation for the observed instability of Mg(TFSI)2 electrolytes.
Research Details
- First-principles and classical molecular dynamics simulations were performed using high-performance computing resources at NERSC.
- Mass transport properties, such as mass diffusion, anodic stability of the electrolyte are evaluated through electrochemical methods.
Work performed at Lawrence Berkeley National Laboratory (JCESR partner) and Argonne National Laboratory (JCESR managing partner) by NN Rajput, X Qu, N Sa, A Burrell and K Persson, Journal of the American Chemical Society, 2015.
DOI: 10.1021/jacs.5b01004

