Scientific Achievement
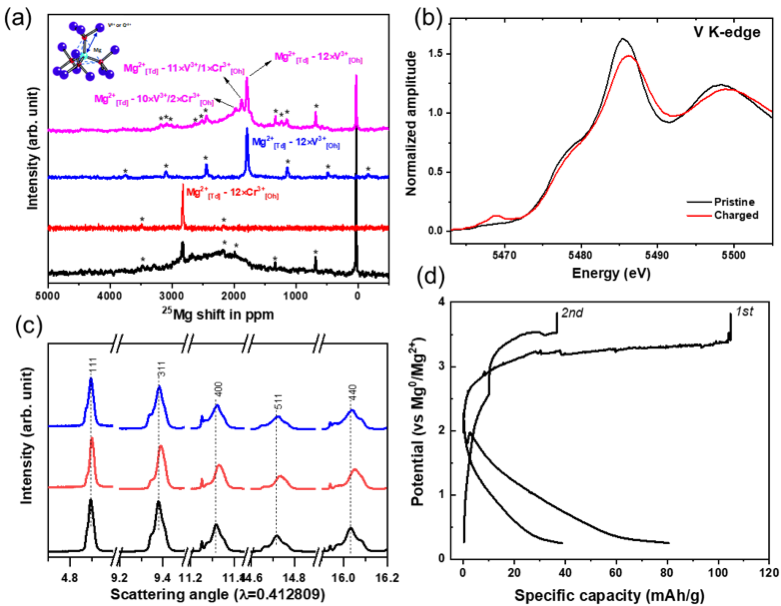
Bulk demagnesiation in MgCrVO4 spinel lattice is observed in a full-cell configuration paired with a Mg metal anode in a chemically and anodically stable Mg(TPFA)2 electrolyte at 110 oC.
Significance and Impact
Solid solution MgCr2-xVxO4 spinel was designed and characterized by theoretical and experimental analyses as a cathode for Mg-ion batteries. We find bulk Mg2+ activity in the designed oxide with partial reversibility in a Mg-ion full-cell configuration. Fundamental mechanisms for reversible Mg2+ intercalation into the high voltage spinel oxides and other irreversible phenomena were proposed by a suite of structural characterization methods. This system provides a step forward in high voltage oxide material design for Mg-ion batteries.
Research Details
- Materials characterization establishing a conclusive proof of bulk Mg2+ reactivity in MgCrVO4 without H+ or H2O co-insertion.
- Mg(TPFA)2 electrolyte at elevated temperatures mitigates kinetic limitations of Mg2+.

