Scientific Achievement
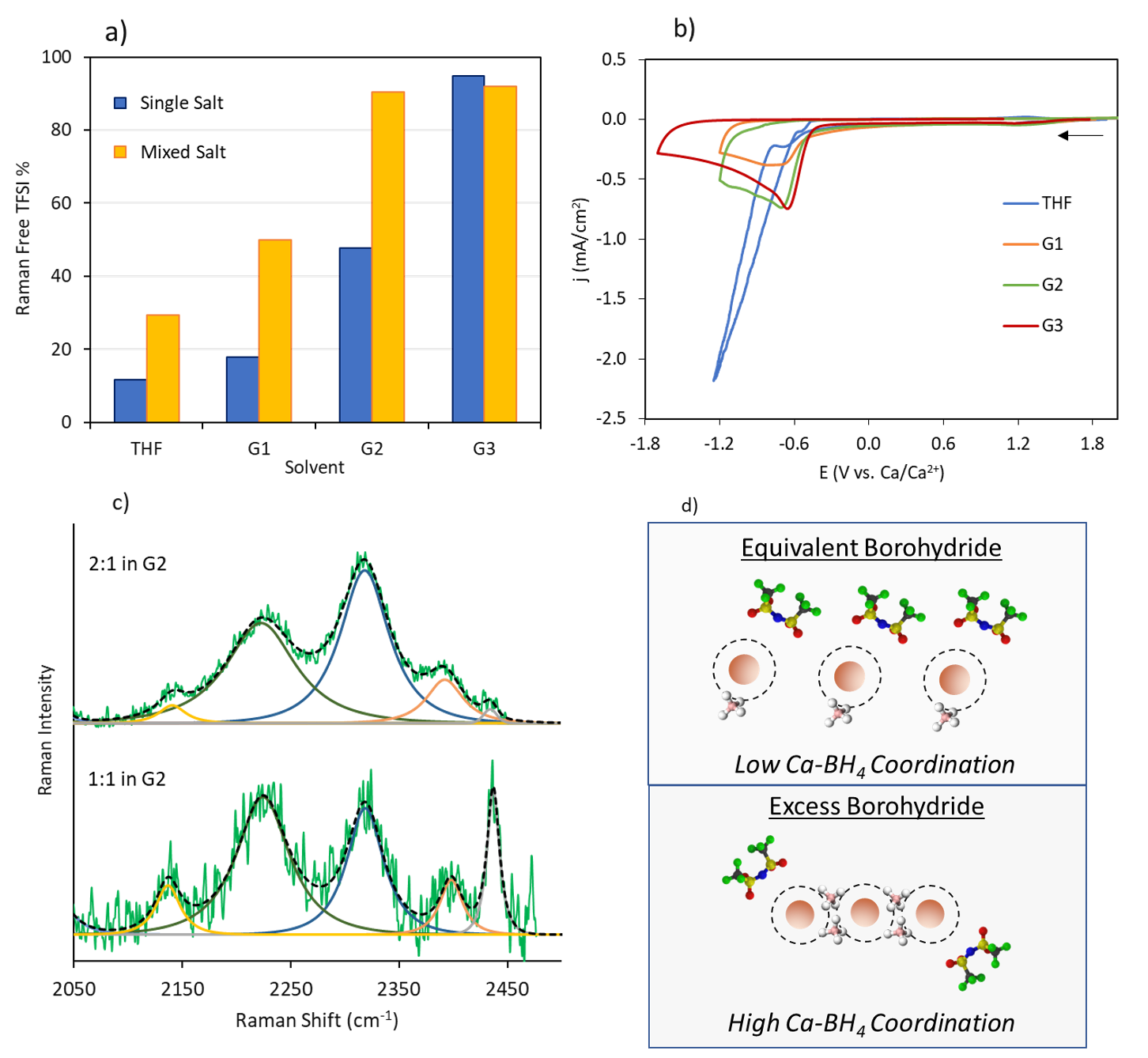
The factors determining the extent to which salt anions can be stabilized during Ca plating by eliminating their coordination with Ca2+ through co-salt addition were elucidated. Contrary to expectation, the exemplar bis(trifluoromethylsulfonyl)imide (TFSI-) anion is unstable whether in the coordinated or free state. Instead, the type of Ca2+ coordination structure formed with the co-salt anion determines whether Ca deposition can be achieved.
Significance and Impact
This work teaches us that eliminating TFSI- coordination is insufficient to prevent TFSI- decomposition at Ca anodes. Interphase design strategies must be employed to cycle Ca at high coulombic efficiency.
Research Details
The influence of adding the strongly coordinating BH4- anion to Ca(TFSI)2 solutions was characterized in four ethereal solvents.
Raman spectroscopy and ionic conductivity measurements indicated that the degree of TFSI- coordination displacement is determined by the solvent’s coordination strength.
Electrochemical Ca deposition trends revealed that the degree of TFSI- coordination displacement is not correlated with the reductive stability of TFSI-.
Assessment of the total coordination environment (TFSI-, solvent, BH4-) revealed that multimer Ca-BH4 coordination structures (shared BH4- ligands) are necessary for stabilizing TFSI-, whether TFSI- coordination is present or not.

