Scientific Achievement
Two important field-wide goals are met through this study: the development of a statistical model for the solubility of conformationally flexible molecules in acetonitrile and the operation of a high concentration (1 M) nonaqueous organic symmetric flow cell.
Significance and Impact
Methods for forecasting nonaqueous solubility would be valuable for streamlining the identification of promising organic electrolytes.
Research Details
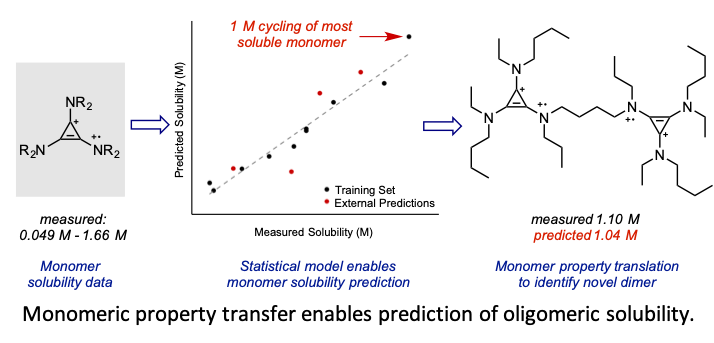
- A statistical, predictive model for solubility in acetonitrile is developed through training on monomeric tris(dialkylamino)cyclopropenium (CP) catholytes.
- The model is demonstrated to effectively translate the model features to enable prediction of CP oligomer solubility. Ultimately, this model is employed to identify a novel CP dimer that is soluble at over 1 M in both redox states, representing a 30% higher charge capacity than the parent monomer.
- The most soluble CP monomer exhibits high stability to electrochemical cycling at 1 M in acetonitrile without supporting electrolyte in a symmetrical flow cell.

