Scientific Achievement
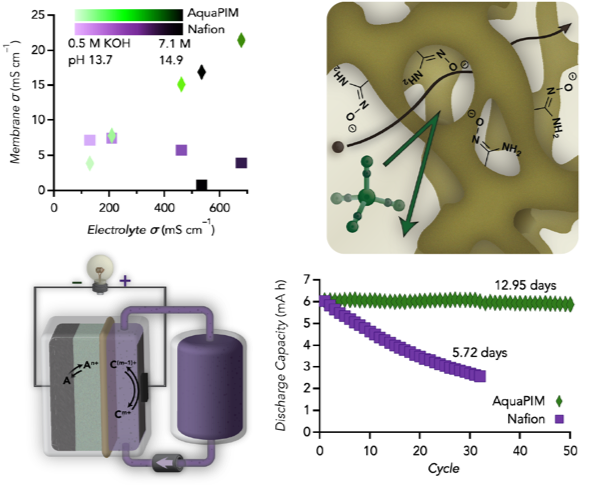
The incorporation of amidoxime units along the rigid backbone of a polymer of intrinsic microporosity to make them aqueous-compatible (AquaPIMs) allows for exceptional conductivity and stability in harsh alkaline environments while blocking a variety of active materials.
Significance and Impact
AquaPIMs can achieve up to 22 mS cm–1 conductivity in highly alkaline electrolytes, outperforming state of the art perfluoroalkylsulfonate membranes.
Research Details
- High conductivity is demonstrated in a variety of electrolytes
- AquaPIM outperforms commercial membranes’ blocking ability for a variety of active materials
- The implementation of AquaPIM membranes in a variety of battery chemistries allows for extended cycle life compared to commercial membranes

