Scientific Achievement
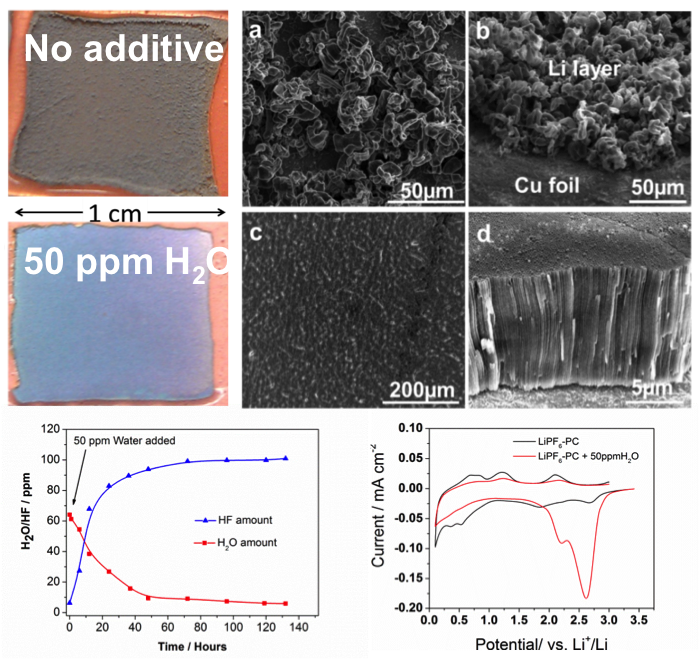
Residual water (H2O) present in nonaqueous electrolytes has been widely regarded as a detrimental factor for lithium (Li) batteries. However, dendrite-free Li film can be obtained with trace H2O as an electrolyte additive.
Significance and Impact
Electrolyte additives are essential to achieve dendrite-free Li metal deposition. Chemical composition of SEI layer strongly affect the growth mechanism and morphology of Li film.
Research Details
- A controlled trace-amount of H2O (25–50 ppm) will react with LiPF6 to form HF
- Detailed analyses revealed that the trace amount of HF will be electrochemically reduced during the initial Li deposition process to form a uniform and dense LiF-rich solid electrolyte interphase (SEI) layer on the surface of the substrate
- The LiF-rich SEI is mechanically stable and can suppress Li dendrite growth
Work performed at Pacific Northwest National Laboratory (JCESR partner), Environmental Molecular Sciences Laboratory by J. Qian, W. Xu, P. Bhattacharya, M. Engelhard, W. Henderson, Y. Zhang and J. G. Zhang, Nano Energy., 2015, 15, 135-144.
DOI: 10.1016/j.nanoen.2015.04.009

