Scientific Achievement
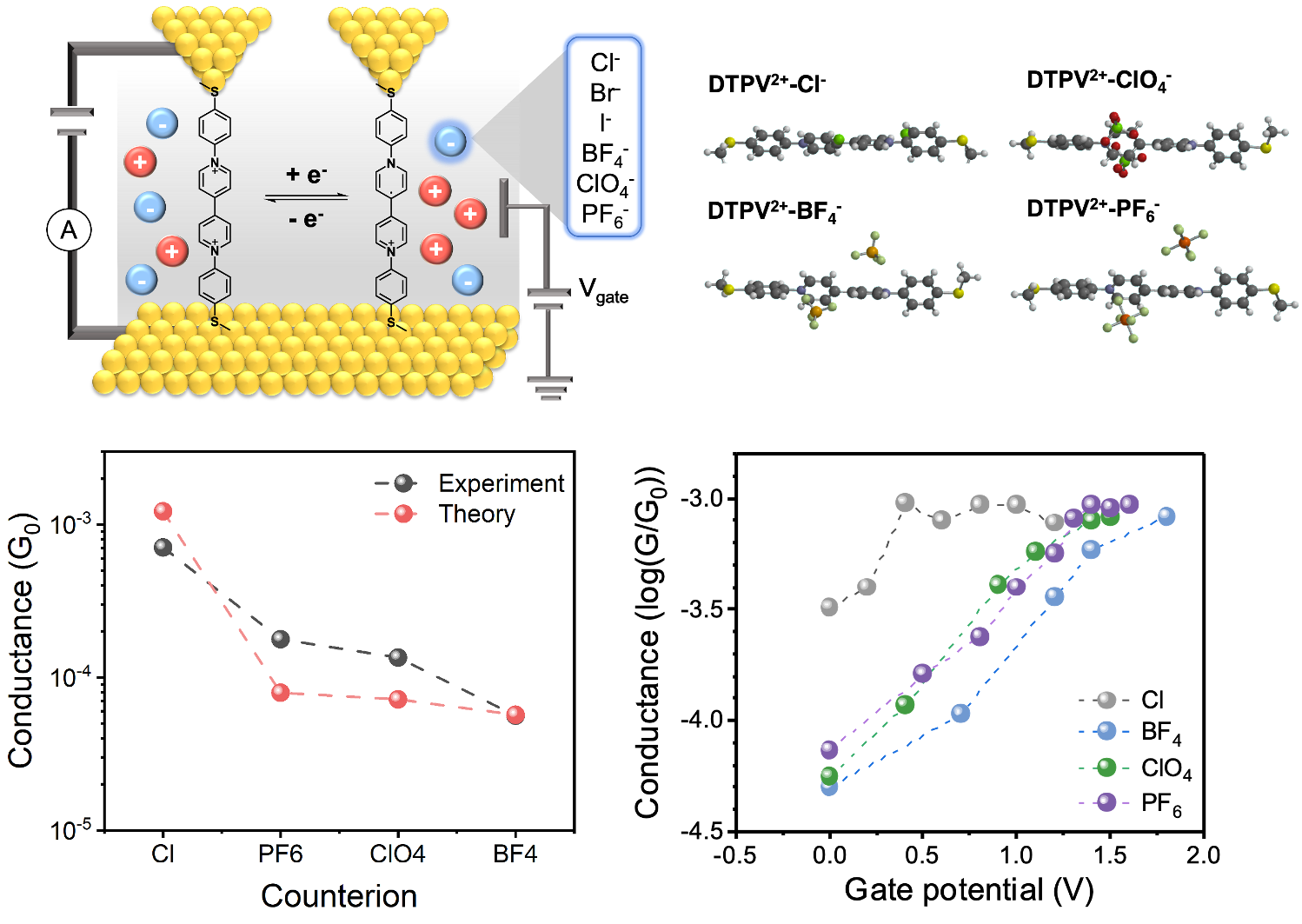
Our results show that charge transport in viologens critically relies the electrochemical environment and is particularly sensitive to the electrolyte counteranion. We use an electrochemical gating technique to understand charge transport in viologens as a function of the redox state and electrochemical environment. Molecular modeling shows that differences in charge transport arise from counterion-induced variations in molecular geometries.
Significance and Impact
This work provides fundamental understanding of how the solvation environment impacts charge transport and underpins the importance of choosing supporting electrolytes. Our work highlights the use of single molecule techniques to understand charge transport in redox-active molecules at electrode interfaces.
Research Details
- Charge transport in viologens is studied using an electrochemical scanning tunneling microscope-break junction method
- Our results show that viologen paired with Cl- shows the highest conductance at 2+ state compared with other counteranions. DFT calculations show that Cl- induces smaller distortions between pyridinium rings and thus leads to higher conductance
- Viologen demonstrates a large and reversible conductance enhancement between 2+ and radical cation state. Molecular planarization contributes to the increasing conductance

