Scientific Achievement
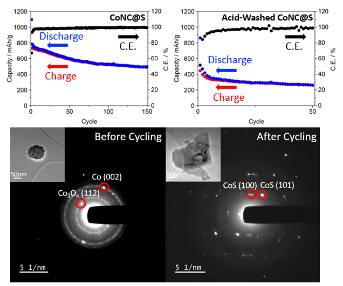
A MOF-derived Co nanoparticle-embedded nitrogen-doped porous carbon (CoNC) was utilized as a catalytic sulfur cathode host for Na-S battery. Postmortem characterization suggests that Co nanoparticles convert to CoS during cycling, and CoS is responsible for catalytic activity on sulfur.
Significance and Impact
Our study provides insights to understanding the role Co-based catalysts play in sulfur batteries and highlights an opportunity to explore cobalt sulfides/carbon composites as catalytic sulfur host for high-performance Na-S batteries.
Research Details
- A MOF-derived Co-containing nitrogen-doped porous carbon (CoNC) was utilized as a catalytic sulfur cathode host
- A NaFSI/DME/BTFE concentrated electrolyte was used.
- Significant improvement in reversible sulfur conversion and capacity retention is observed with higher Co-content in CoNC.
- Postmortem XPS, TEM and selected area electron diffraction (SAED) suggests that CoS is formed during cycling in place of Co nanoparticles and CoN4 sites.
- Raman spectroscopy suggests that CoS exhibits a catalytic effect on the oxidation of Na2S.

