Scientific Achievement
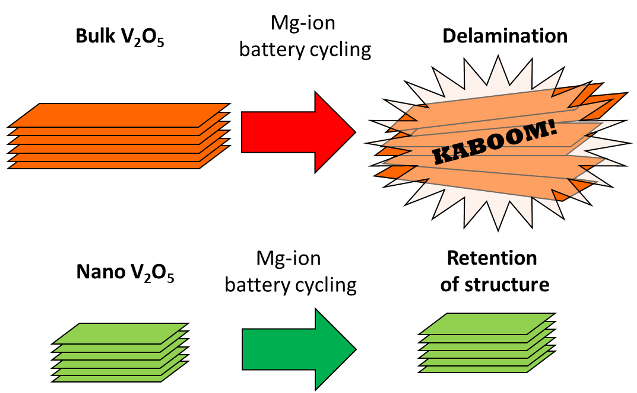
Reducing the particle size of α-V2O5 dramatically improved the energetics of Mg intercalation on the first cycle, whereas latter cycles showed no relative benefits, revealing that non-diffusive barriers contribute to current limitations in Mg batteries
Significance and Impact
This study reveals the impact of diffusive barriers on Mg-based energy storage, and indicates that exploration of non-diffusive limitations within Mg battery cycling are necessary to develop an energy dense Mg battery.
Research Details
- Incorporation of a nanometric TiO2 seed reduced the size of α-V2O5 crystallites from 140 nm to 25 nm.
- The 25 nm α-V2O5 crystallites displayed much lower voltage hysteresis (1.05 V) compared to 140 nm α-V2O5 (1.68 V) on the first cycle, but little difference was observed on latter cycles.
- Structural, redox and elemental changes in α-V2O5 with Mg insertion and removal were confirmed by JCESR collaborators within the Materials Complexity Thrust.

