Scientific Achievement
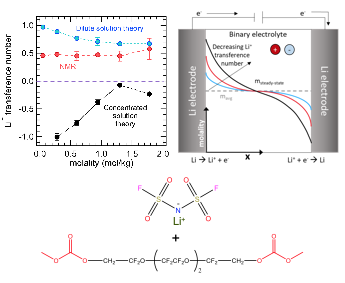
Common approaches to measuring the lithium-ion transference number for fluorinated electrolytes leads to severe over-estimation of this value.
Significance and Impact
The disparity between the three transference numbers, which indicates the dominance of ion clustering, is resolved by the use of Newman’s concentrated solution theory. Properly measuring the Li+ transference number allows for accurate determination of salt concentration gradients in batteries as large concentration gradients lead to battery failure.
Research Details
- The steady-state current method, NMR and Newman’s concentrated solution theory were each used to determine the Li+ transference number of a fluorinated electrolyte as a function of salt concentration
- The conductivity, salt diffusion coefficient, Li+ transference number, and the thermodynamic factor were all determined experimentally

