Scientific Achievement
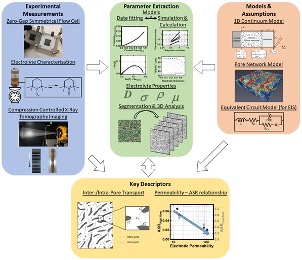
Through a combined experimental and modeling approach, we show that, of the many physical properties associated with fibrous electrodes, permeability best correlates with electrochemical performance in a flow cell.
Significance and Impact
The impact of electrode choice on cell performance can be difficult to predict a priori. This work shows permeability and mass-transfer coefficients can be used to predict performance of different porous materials as redox flow battery electrodes.
Research Details
- Four distinct carbon electrodes are tested in a redox flow cell using TEMPO, a kinetically facile redox couple, dissolved in two different electrolytes which result in solutions with different viscosities and conductivities.
- Three-dimensional microtomographic renderings of the different electrodes are used to extract morphological data from which permeability and tortuosity tensors can be extracted via pore network modeling and direct numerical simulation.
- A one-dimensional electrode model is used to fit the experimental flow cell data to obtain mass-transfer coefficients in the different electrolyte environments.
DOI: https://doi.org/10.1016/j.apenergy.2021.117678

