Scientific Achievement
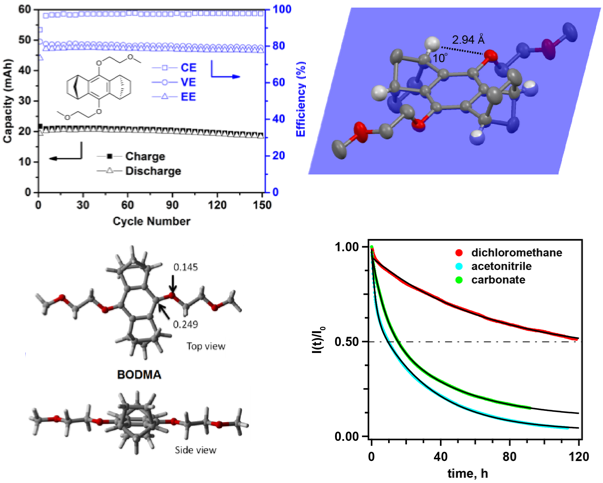
9,10-bis(2-methoxyethoxy)-1,2,3,4,5,6,7,8- octahydro-1,4:5,8-dimethanenoanthracene (BODMA) was developed for use as the catholyte in non-aqueous redox flow batteries. The bicyclic scaffolds prevent the ring-addition reaction, showing superior chemical stability in the charged state. A hybrid flow cell using this catholyte is operated for 150 charge-discharge cycles with a minimal loss of capacity.
Significance and Impact
BODMA fully prevents the dominating decomposition pathway of previous 1,4-dialkoxybenzene-based catholytes while retaining high battery compatibility. The exceptional performance of this material in the cycling tests places our redox system among the most promising flow cell chemistries ever reported.
Research Details
BODMA delivered a reversible redox potential of 4.0 V vs Li/Li+, a half life time of 120 hours in the charge state, and a stable performance over 150 cycles in a hybrid flow cell.
- Single crystals and DFT calculations indicated that the constrained conformation of the bicyclic substituents is the key to maintain the electrochemical reversibility of BODMA.

