Scientific Achievement
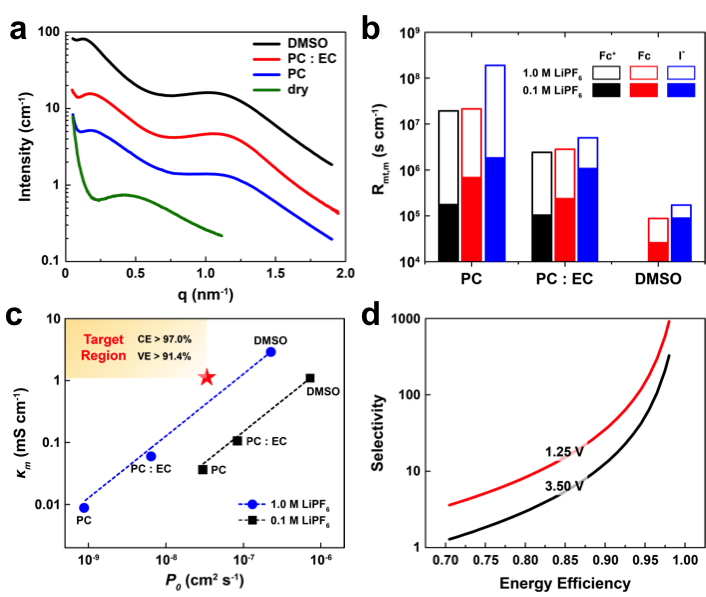
Solvent volume fraction within the membrane has the greatest effect on both conductivity and crossover; as a secondary effect, the charge on redox species modifies crossover rates in accordance with Donnan exclusion.
Significance and Impact
The relatively low conductivities or selectivities of Nafion under nonaqueous conditions suggest that new membranes are required before a competitive nonaqueous RFB could be implemented.
Research Details
- Li-N117 was used as a model ion-exchange membrane in various nonaqueous solvents/electrolytes.
- The structural information of the membranes was characterized using solvent hydraulic permeability tests and X-ray scattering.
- The membrane conductivity and active species crossover were measured and correlated to the target performance.
Work performed at Massachusetts Institute of Technology (JCESR collaborator), United Technologies Research Center (JCESR collaborator), Argonne National Laboratory (JCESR managing partner), University of California, Lawrence Berkeley National Laboratory (JCESR partner), and University of Illinois at Urbana-Champaign (JCESR partner) by L. Su, R. M. Darling, K. G. Gallagher, W. Xie, J. L. Thelen, A. F. Badel, J. L. Barton, K. J. Cheng, N. P. Balsara, J. S. Moore, and F. R. Brushett. J. Electrochem. Soc. 163, (2016)

