Scientific Achievement
- A series of methyl-substituted 1,4-dimethoxybenzene derivatives were developed via a subtractive design approach through reengineering of DBBB.
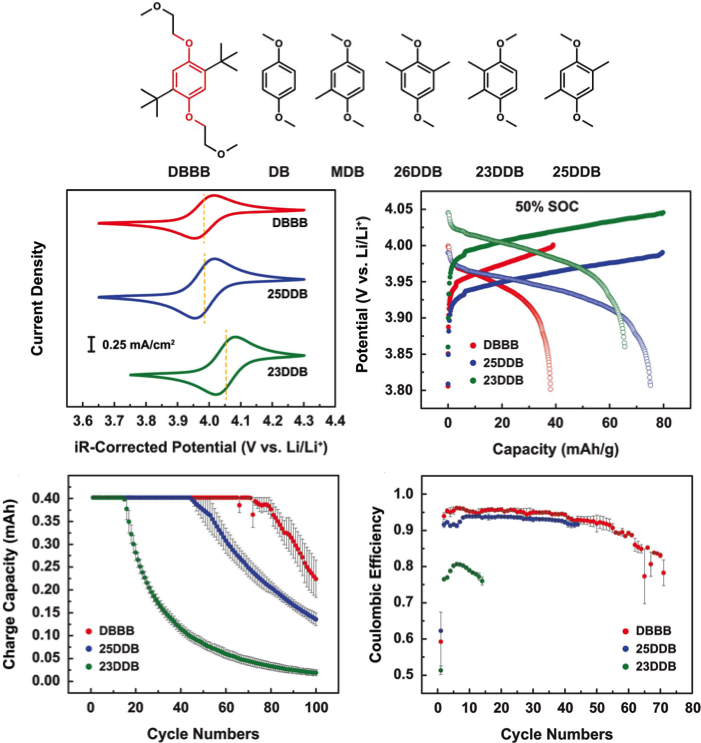
- 23DDB and 25DDB demonstrated desirable electrochemical and physical characteristics for nonaqueous redox flow batteries with intrinsic capacities at 161 mAh/g.
Significance and Impact
- Our examination indicated that 23DDB and 25DDB may set the upper limit on the intrinsic capacity realistically achievable for 1,4-dimethoxybenzene derivatives.
- Further increase of the intrinsic capacity is likely to require the involvement of reversible two-electron reactions to meet the rather aggressive cost target of $100/KWh.
Research Details
- Five molecules were proposed, three of them were synthesized and characterized by NMR and GCMS.
- Cyclic voltammetry, diffusivity, solubility and galvanostatic cycling with commercially available bulk electrolysis cell were used to evaluate the electrochemical and physical properties of the proposed molecules for nonaqueous redox flow batteries.
Work performed at Argonne National Laboratory (JCESR managing partner) and Massachusetts Institute of Technology (JCESR collaborator)Huang, L. Su, J. A. Kowalski, J. L. Borton, M. Ferrandon, A. K. Burrell, F. R. Brushett, L. Zhang, J. Mater. Chem. A. 2015.
DOI: 10.1039/C5TA02380G

