Scientific Achievement
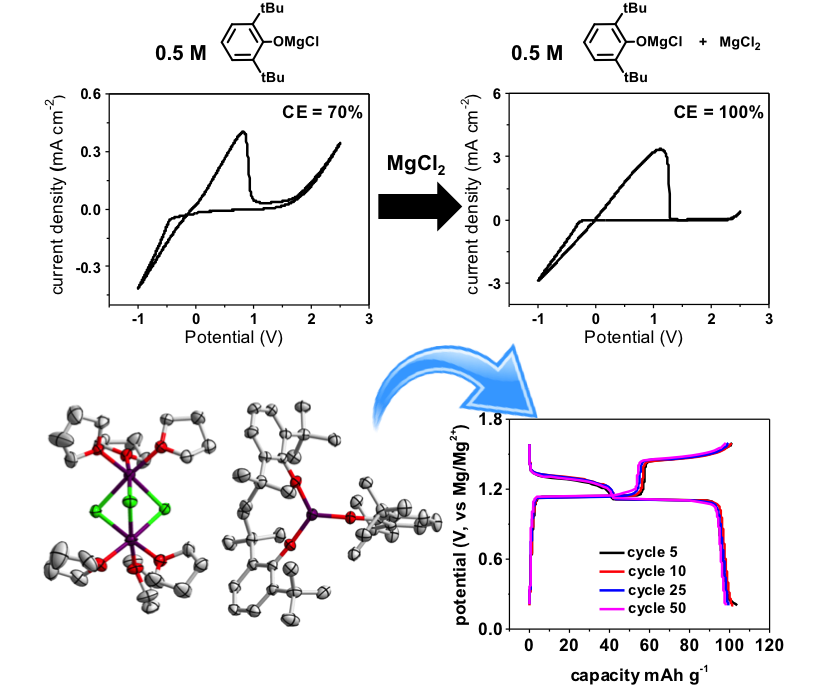
A novel Lewis acid-free all magnesium electrolyte containing 2,6-di-tert-butylphenoxidemagnesium chloride ((DTBP)MgCl + MgCl2) has been deliberately developed. The obtained electrolyte possesses the oxidative stability of up to 2.3 V (vs Mg/Mg2+) with 100% coulombic efficiency, which is comparable to its AlCl3 counterpart.
Significance and Impact
Instead of using corrosive and toxic strong Lewis acid AlCl3, environmentally benign MgCl2 has been used to improve the oxidative stability of this phenoxide-based magnesium electrolyte system. This strong and corrosive Lewis acid-free strategy opens a new door to the development of more highly oxidative stable magnesium electrolytes.
Research Details
Addition of benign salt MgCl2 into (DTBP)MgCl in THF solution results in the significant improvement of the oxidative stability from less than 1.5 V to 2.3 V. The excellent battery cycling performance using Chevral phase Mo6S8 further demonstrates the great potential of our electrolyte for rechargeable magnesium-ion batteries.
Work performed at Argonne National Laboratory (JCESR managing partner) by B. Pan, J. Zhang, J. Huang, J. T. Vaughey, L. Zhang, S-D. Han, A. K. Burrell, Z. Zhang and C. Liao, Chem. Commun. 2015
DOI: 10.1039/C5CC01225B

