Scientific Achievement
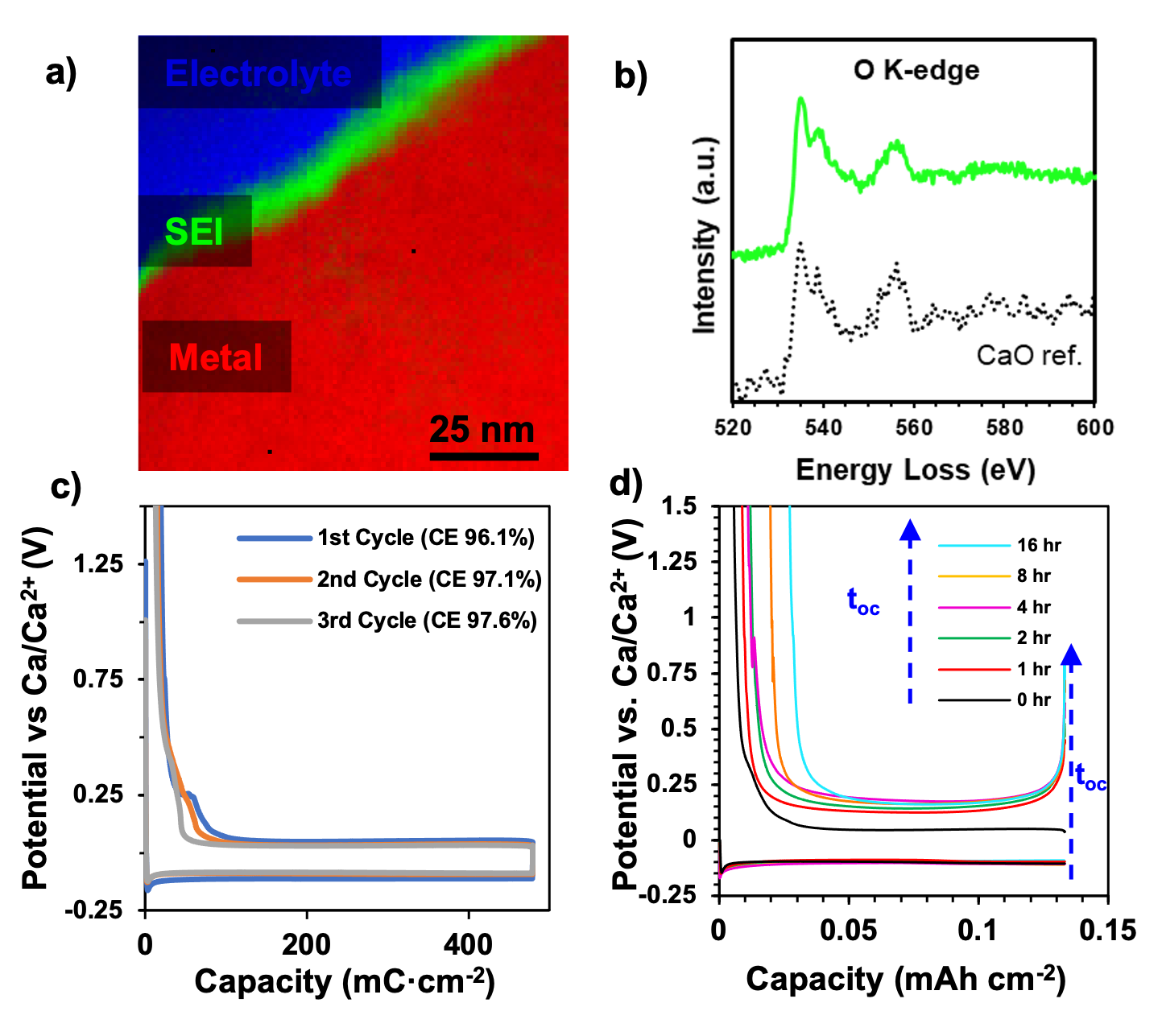
The solid electrolyte interphase (SEI) formed on the calcium anode during reversible calcium electrodeposition has been compositionally mapped revealing the role of a heterogenous, nanometric calcium oxide as the responsible cation conductor and protective interphase.
Significance and Impact
Our ability to correlate interphase identity and electrochemical performance with the multivalent cation solvation structure of the electrolyte enables enhanced battery performance through electrolyte design.
Research Details
A cryogenic workflow ensures intact SEI transfer from the electrochemical cell to the transmission electron microscope.
Cryo-electron energy loss spectroscopic (cryo-EELS) mapping provides an unambiguous determination of the SEI as a compositionally heterogeneous calcium oxide containing calcium borate and calcium carbonate.
From parallel structural mapping, we propose grain and phase boundaries are responsible for Ca2+ transport.
DFT computation highlights the origin of the interphase as decomposition of a solvent separated calcium – borohydride ion pair unique to the electrochemical double layer.
This interphase is demonstrated as responsible for high coulombic efficiency Ca plating and stripping, as well as retarding self-discharge during inactive periods.

