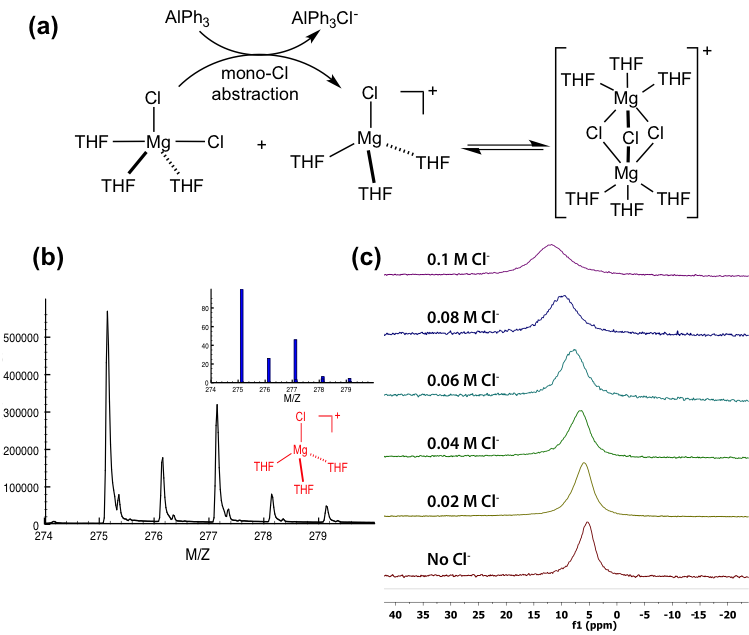
(b) The M/Z isotopic distribution of the [MgCl(THF)3]+ peak in the positive mode of SPIN MS of [(µ-Cl)3Mg2(THF)6]AlPh3Cl. Inset gives the calculated isotopic pattern
(c) 25Mg NMR spectra of [(µ-Cl)3Mg2(THF)6]AlPh3Cl in the presence of tetrabutylammonium chloride (TBACl)
Scientific Achievement
- First characterization of the long-sought [MgCl]+ species of the [(μ-Cl)3Mg2(THF)6]+ dimer electrolytes using a soft mass spectrometry, Subambient Pressure Ionization with Nanoelectrospray Mass spectrometry (SPIN-MS)
- Electrochemical, SPIN-MS, and 25Mg NMR studies of the coordination interactions of exogenous Cl– with the dimer electrolytes
Significance and Impact
- The [(μ-Cl)3Mg2(THF)6]+ dimer has a dynamic equilibrium and requires a special tool to identify key species that can be achieved by the SPIN-MS technique.
- The pinpointed [MgCl]+ species and the supporting electrochemical, Raman, and 25Mg NMR studies enable a comprehensive understanding of the coordination chemistry and Mg2+ cycling mechanism of the dimer electrolytes.
Research Details
- SPIN-MS was adopted to identify the [MgCl(THF)3]+ species of a series of [(μ-Cl)3Mg2(THF)6]+ dimer electrolytes.
- Raman studies confirmed the existence of [MgCl2(THF)x] and the [(μ-Cl)3Mg2(THF)6]+ dimer.
- The coordination interactions of Cl– with the dimer electrolyte were studied and analyzed using a variety methods.
Work performed at Pacific Northwest National Laboratory (JCESR partner) by Tianbiao Liu, Jonathan T. Cox, Dehong Hu, Xuchu Deng, Jianzhi Hu, Mary Y. Hu, Jie Xiao, Yuyan Shao, Keqi Tang and Jun Liu, Chem. Commun.,2015.
DOI: 10.1039/C4CC07621D

