Scientific Achievement
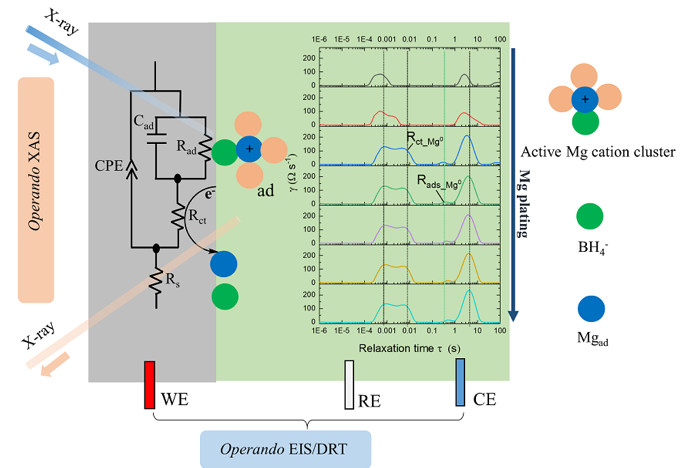
A detailed description of the complex charge-transfer process at a Mg/electrolyte interface is provided where an additional adsorption process, during Mg plating, is confirmed to be a key step.
Significance and Impact
This study demonstrates a comprehensive approach to probing and understanding electrified electrode/electrolyte interfaces where the discovery and understanding of new adsorption chemistries are critical for the predictive design of a reversible interface.
Research Details
- A critical adsorption step between Mg0 atoms and active Mg cation clusters involving BH4− anions is identified to be the key enabler for reversible Mg plating/stripping.
- Addition of BH4− leads to the neutralization of the first solvation shell of Mg cationic clusters between Mg2+ and TFSI- and enhanced reductive stability of free TFSI-.
- A new interpretation method (see Figure), deconvoluting operando EIS data, is demonstrated to be a novel and feasible approach to probe and understand the electrified interface, especially when coupled with operando soft XAS experiments.

