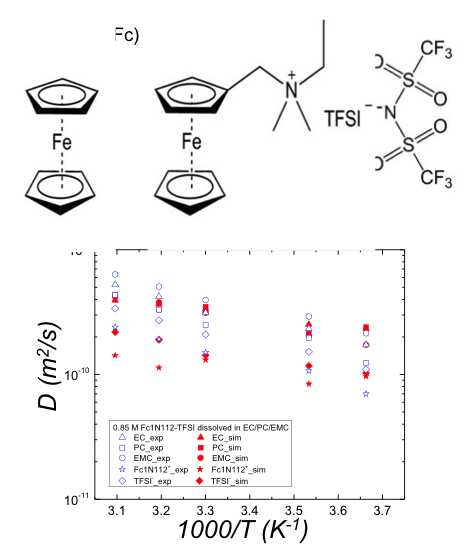
(Bottom) Experimental and simulated self diffusion coefficients (D) for solution components as a function of temperature
Scientific Achievement
A coupled experimental and computational study of the structure and mobility of ferrocene (Fc) and a derivitized Fc-based ionic compound (Fc1N112-TFSI, see figure for structure) reveals the differences at the molecular level that explain improved electrochemical behavior.
Significance and Impact
- A molecular-level explanation has been gained explaining why the solubility of Fc/Fc+ redox centers can be increased up to eight-fold in a mixture of carbonate electrolytes through the modification of Fc with a polar tetra-alkylammonium functional group with a bis(trifluoromethanesulfonyl)-imide anion.
- The higher effective viscosity and activation energy for translation when Fc1N112-TFSI is dissolved in a mixed solvents (EC/PC/EMC) show that the interaction between PC and Fc1N112+ is the strongest among the three components of the solvent.
- Results from MD simulations confirm the experimental results and provide molecular-scale insight into the translational and rotational dynamics.
Research Details
- The diffusion coefficients D of solutes and solvent molecules were measured by 1H and 19F pulsed field gradient (PFG) nuclear magnetic resonance (NMR) in the temperature range of 0~50 °C and for various concentrations of Fc and Fc1N112-TFSI. The three components of the mixed electrolyte (EC, PC and EMC) have the same diffusion coefficients in a neat solution and when 0.2 M Fc is dissolved in EC:PC:EMC, but when Fc1N112-TFSI is dissolved in the mixed solvents, measurements show that DPC<DEC<DEMC.
- Classical MD simulations were performed in the temperature range of 0~50 °C and for various concentrations of Fc1N112-TFSI in pure PC, pure EMC and a mixed solvent system of EC/PC/EMC under constant NVT ensemble.
Work performed at Pacific Northwest National Laboratory (JCESR partner) and Lawrence Berkeley National Laboratory (JCESR partner) by K. S. Han, N. N. Rajput, X. Wei, W. Wang, Jian Zhi Hu, K. A. Persson and K. T. Mueller, J. Chem. Phys. 141 (2014) 104509.
DOI: 10.1063/1.4894481

