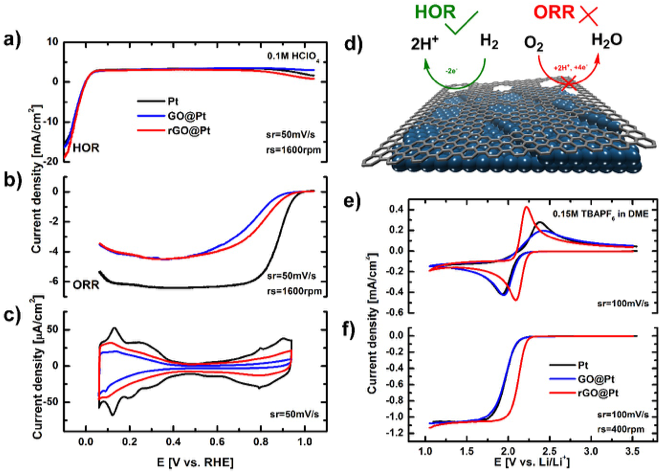
Scientific Achievement
A modified Langmuir-Blodgett approach is developed for the deposition of GO and rGO films that enables ex situ engineering of the activity and selectivity of electrochemical interfaces in both aqueous and non-aqueous electrolytes.
Significance and Impact
This methodology points to a new strategy for introducing intentional interphase layers at liquid-solid interfaces that directly impact their (electro)chemical response.
Research Details
- Both GO and rGO interfaces on Pt electrodes selectively inhibit the oxygen reduction reaction (ORR), but they do not have an impact on the activity of the hydrogen oxidation reaction (HOR) in acidic environments.
- Selectivity in aqueous media is attributed to the presence of defects in GO and rGO that allow H+ diffusion to the underlying Pt electrode while suppressing OH- transport.
- rGO interfaces are further demonstrated to exhibit two orders of magnitude enhancement in activity for the non-aqueous ORR.
- Removal of GO oxygen surface functionalities upon transformation to rGO likely modify the double layer structure, leading to enhanced ORR activity on rGO surfaces in non-aqueous electrolytes.

