Scientific Achievement
The electrolyte of the lithium-sulfur (Li-S) battery was tuned to both overcome the need for a high electrolyte/sulfur ratio and inhibit the lithium dendrite growth on the anode at the same time.
Significance and Impact
Achieving a Li-S battery that operates for numerous charge-discharge cycles with a minimal amount of electrolyte is an important step in the development of low-cost, high energy density batteries.
Research Details
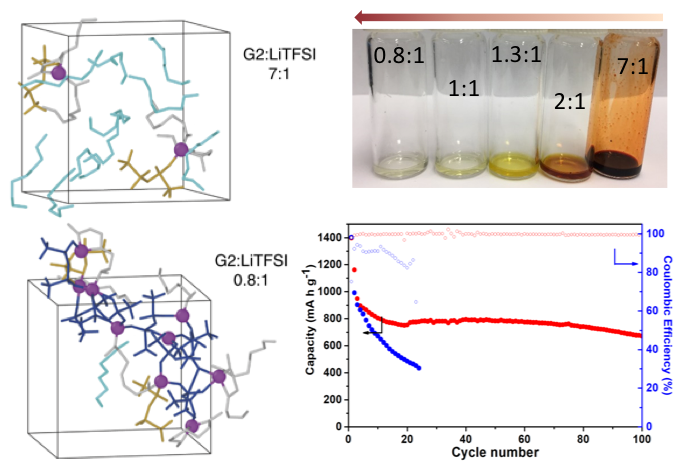
- This new electrolyte consists of an ether (“diglyme”) solvent with a lithium salt, where the solvent/salt ratio is tuned to minimize the solvent activity.
- This tuning transforms the sulfur reaction pathway at the cathode in such a way that the related polysulfide dissolution reactions occur partially in the solid state, as opposed to just in the liquid electrolyte.
- As shown by experimental and computational studies, the resulting lowered activity for the solvent along with an extended electrolyte network structure curtail the need for high electrolyte volumes.
- In addition, the optimized electrolyte structure avoids the problem with electrolyte solvent degradation by suppressing dendrite formation, greatly improving cycle life.
DOI: 10.1038/s41560-018-0214-0

